History
A 22-year-old white male presented with a chief complaint of decreased vision, which had progressively worsened during the past two years.
 |
Several previous eye care providers informed the patient that he needed to see a retina specialist. Because of insurance-related complications, however, he never sought specialized retinal care.
The patient had no documented ocular history. He appeared to be in good systemic condition, and said that he didn’t take any medications. Further, he reported no known allergies of any kind.
Diagnostic Data
Best-corrected visual acuity measured 20/80 OU at distance and 20/50 OU at near. External examination uncovered some central distortion on facial Amsler testing, which was confirmed on grid assessment.
We documented no evidence of afferent pupillary defect OU, but red color interpretation was unequal between the two eyes. There was no brightness desaturation. Refraction uncovered no changes OU.
The anterior segment evaluation was normal in both eyes. Intraocular pressure measured 16mm Hg OU. The pertinent posterior segment findings are illustrated in the photographs.
Your Diagnosis
How would you approach this case? Does this patient require any additional tests? What is your diagnosis?
How would you manage this patient? What’s the most likely prognosis?
Discussion
Additional testing included Ishihara color vision evaluation to rule out maculopathy and optic neuropathy. The patient correctly identified 13/14 plates OD and 14/14 plates OS. Red cap and brightness testing was also completed to rule out optic nerve dysfunction. Results to both tests were negative. Finally, we performed an automated visual field screening, which revealed a superior nasal defect OD.
The fundus photograph revealed choroidal folds in the right eye. Optical coherence tomography (OCT) confirmed a serous retinal detachment in the right eye, resulting in an emergent referral to retinology. Given the finding of choroidal folds and the potential for a space-occupying lesion, we performed Hertel exophthalmometry (21mm x 21mm with a 105 base) and referred her for a neuro-ophthalmology work-up. Neuroimaging studies were ordered––specifically an MRI of the brain and orbits, both with and without contrast––to rule out an orbital mass.
The MRI did not reveal any mass in the orbit or brain; however, it did show thickening and flattening of the globe’s posterior wall (OD > OS), indicating the presence of inflammation or infiltration. The comanaging retinologist performed a fluorescein angiography (FA), which demonstrated diffuse multifocal hyperfluorescent areas of leakage that increased in intensity over time. B-scan ultrasonography was performed and showed thickening of the posterior globe and sonolucency in the region of the sclera that corresponded to the area of subretinal fluid. There was no evidence of fluid in the Tenon’s space.
The diagnosis in this month’s issue is posterior scleritis––a rare condition that can mimic a variety of ocular inflammatory and neoplastic conditions.1 It is one of the more difficult disorders to diagnose due to its low incidence, location and varied clinical presentation.1 It must be considered as part of the differential diagnosis of many ocular conditions, including angle-closure glaucoma, choroidal folds, optic disc edema, circumscribed fundus mass, choroidal detachment, exudative retinal detachment and carotid cavernous fistula.2-4
Posterior scleritis typically presents unilaterally, and has an average age of onset of 50 years.5,6 It affects twice as many females as males, more adults than children and more whites than non-whites.6,7 The older the patient, the more likely he or she is to experience associated vision loss.6 All patients with posterior scleritis are at risk for permanent visual compromise; therefore, early treatment is essential to control inflammation and subsequently preserve vision.
Inflammation secondary to posterior scleritis may remain localized to a specific area of the posterior segment.8 In other instances, however, it can spread anteriorly and mimic orbital cellulitis.9 One study group found that 40% of patients had no anterior scleritis––some of which exhibited no detectable physical signs.6
Because the anterior segment isn’t always involved, external signs of inflammation, such as red eye, may not be noted. Additionally, decreased visual acuities and visual fields may be noted.3,6,7 Inflammation from posterior scleritis also has the potential to spread and involve other orbital structures or manifest more diffusely posteriorly. It is worth noting that diplopia may occur if the inflammation restricts eye movement.5
Most patients will complain of some degree of pain, ranging from mild distress upon eye movement to significant discomfort that prevents sleep. This pain may radiate to the surrounding structures, yielding referred pain from the brow and jaw.5,7 The variability and location of the pain often causes posterior scleritis to be misdiagnosed as sinusitis or migraine headache.7 An anterior or posterior uveitis is present in 70% of cases.7 A shallowing of the anterior chamber and/or glaucoma also may be present. Further, intraretinal lesions resembling hard exudates, as well as pigment epithelial elevations and mottling of the pigment epithelium, all have been associated with posterior scleritis.7
Because there are no pathoneumonic signs or symptoms for posterior scleritis, ancillary testing often is essential for diagnosis. B-scan ultrasonography should be performed on all patients who exhibit choroidal folds of an undiagnosed origin.6,10 It is important to note that posterior scleritis frequently causes eye well flattening and thickening (greater than 2.0mm).2,11
Edema often is present in Tenon’s space.12 This clinical presentation is referred to as the “T sign,” because it is formed by a squaring of the normally rounded optic nerve shadow with the edema that extends along the adjacent sclera.4,13,14 This configuration creates the top of the “T,” with the nerve itself creating the stem. (Mild posterior scleritis may not yield a T sign.14) Additionally, optic disc swelling, distended optic nerve sheath, retinal detachment and scleral nodules also can be noted on ultrasound.7,11
An A-scan can help differentiate between posterior scleritis and a malignant choroid melanoma. Posterior scleritis typically reveals high internal reflectivity associated with the scleral thickening, whereas a malignant choroidal melanoma is associated with low to medium internal reflectivity.3,10,12
In questionable cases, FA is valuable in distinguishing between central serous chorioretinopathy and choroidal melanoma.14 Posterior scleritis may show blocked fluorescence in the arteriovenous phase and diffuse hyperfluorescence in the late phase, with no leakage. Choroidal folds are seen as an alternation of hyperfluorescent and hypofluorescent bands on FA, helping to distinguish choroidal folds from retinal striae.14 In the case of nodular posterior scleritis, normal choroidal circulation and a lack of “double circulation” is noted via FA.11
Indocyanine green (ICG) angiography also can be used to identify areas of inflammation. It is useful in assessing the extent of choroidal involvement, as well as the response to the therapy. Patients with posterior scleritis will show hyperfluorescence in the intermediate and late phases of ICG angiography.15 Dark, irregularly distributed dots also may be detected during the intermediate phase; these will become isofluorescent in the late phase, lending a mottled appearance to the choroid.16
With the advent of OCT, the details of the retinochoroidal structures may be visualized in cross section, revealing the extent of inflammation due to posterior scleritis. The instrument can uncover serous retinal detachment or other structural changes.8 On OCT, choroidal folds exhibit a wavy appearance, and the instrument has the capability to distinguish if the folds are due to vitreoretinal traction or the presence of a subretinal mass.17 OCT also can be used to monitor the progress of treatment.18
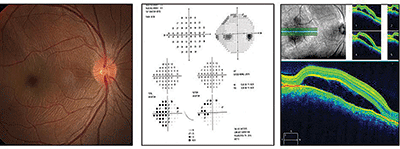
|
|
| Fundus image, visual fields test and spectral-domain optical coherence tomography scan of our 21-year-old patient's right eye. What do you notice, and how should she be managed? |
Computerized tomography (CT) and magnetic resonance imaging (MRI) with and without contrast can be used to visualize characteristic scleral thickening and the T sign.19 However, due to its higher resolution capacity, ultrasound is preferred over a CT scan.1 It is important to note that orbital pseudotumor can demonstrate similar ultrasound and CT scan findings, but without the presence of anterior uveitis, optic disc edema, subretinal mass and choroidal folds. In some cases, MRI is useful for differentiating posterior scleritis from orbital inflammation, neoplasms, extraocular muscle or lacrimal gland enlargement.17 These tests are important when proptosis is present or if carotid-cavernous fistula is suspected.4
Cases of nodular posterior scleritis can be particularly challenging. They can present similarly to choroidal and amelanotic melanoma.3,19 Fortunately, the condition is uncommon (just 1.5% of lesions that simulate choroidal melanoma are precipitated by nodular posterior scleritis).3,19 Misdiagnosis can be avoided in questionable cases via scleral trephine punch biopsy of the presumed mass.20 A biopsy also can be used to determine the cellular reaction at the site (i.e., inflammatory vs. infective).21 This measure only is considered in extreme cases, because the sclera does not heal well.5
Posterior scleritis is predominantly idiopathic or autoimmune.6 One retrospective review indicated that 29% of patients had an associated systemic disease, including systemic vasculitis, lymphoma and autoimmune disease (most commonly rheumatoid arthritis).6,11 The older the patient, the more likely that posterior scleritis is associated with a systemic disease. On rare occasions, especially when the condition is an extension of anterior scleritis, it also may be caused by an infectious entity. Several bacteria, fungi and parasites have been linked to scleritis––usually after patients undergo cataract surgery, scleral buckling surgery, pterygium surgery, suture removal or scleral buckle removal, as well as following trauma or systemic infection.21
The workup for unexplained cases of posterior scleritis should include rheumatoid factor; antinuclear antibodies; antineutrophil cytoplasmic antibodies; human leukocyte antigen typing; eosinophil count/immunoglobulin; erythrocyte sedimentation rate; hepatitis B surface antigen; serologies for infectious diseases, such as syphilis and Lyme; purified-protein derivative; anergy skin testing; and chest, sinus, sacroiliac and limb radiographic studies.15
A complication of posterior scleritis potentially can be ciliochoroidal effusion syndrome. This refers to an abnormal collection of fluid between the sclera and the choroid or ciliary body that expands the suprachoroidal space, thereby producing internal choroidal elevation. Although a ciliochoroidal effusion usually is associated with low intraocular pressure, it may sometimes result in anterior movement of the iris and ciliary body, secondary angle-closure glaucoma and elevated intraocular pressure. The treatment for this secondary angle closure due to ciliochoroidal effusion syndrome differs from primary angle closure with pupillary block.
In the case of ciliochoriodal effusion syndrome, it is important to relieve the iris compression of the angle by displacing the lens iris diaphragm posteriorly via cycloplegia. Intraocular pressure-lowering agents should include fast-acting topical medications, such as timolol and apraclonidine BID, and oral agents, such as 500mg acetazolamide QD to BID. Miotics are contraindicated. Topical steroid use is advisable to reduce inflammation.
If left untreated, posterior scleritis is visually threatening.6 Additionally, complications from posterior scleritis may permit the inflammation to spread to muscle or other orbital tissues. Scleral thickening due to posterior scleritis can restrict the opening through which the optic nerve passes, resulting in compression. Displacement of the globe’s posterior wall secondary to inflammation can cause choroidal folds and a hyperopic shift in refraction. Most other ocular complications are due to chronic inflammatory tissue destruction. These include uveitis, cataract formation and retinal detachment due to the accumulation of exudates under the neural retina, as well as scleral thinning and recurrence of inflammation following treatment discontinuation.12 Visual prognosis generally is worse in long-duration cases (i.e., those that persist for more than 10 weeks).12
Treatment of posterior scleritis is not as challenging as its diagnosis. If an etiology is determined, an appropriate treatment is much easier to surmise.12 However, most patients with idiopathic posterior scleritis respond well to nonsteroidal anti-inflammatory medications.4 When inflammation is nonresponsive or a systemic disease is concurrent, more aggressive treatment is required. This may include both systemic corticosteroids and noncorticosteroid immunosuppressive medications, such as methotrexate or azothioprine.13 In certain cases, if more aggressive treatment is warranted, steroidal periocular or intravenous injections may be used.6,11,14 Complete resolution may take several weeks, although choroidal folds may persist even after scleritis resolution.
Our patient’s condition resolved completely following a six-week course of oral NSAID dosing. Her vision also returned to baseline (20/20 OU). No underlying systemic etiology was found, despite extensive and repeated blood work.
Thanks to Heather Miller, OD, of Holland, Pa., and Michael Rebar, Pa., of Coatesville, Pa., for contributing this case.
1. Shukla D, Kim R. Giant nodular posterior scleritis simulating choroidal melanoma.
Indian J Ophthalmol. 2006 Jun;54(2):120-2.
2. Wang Jia-Kang, Lai Pei-Ching, Yang Chang-Hao. Subretinal mass as a presenting sign of posterior scleritis: A case report. Kaohsiung J Med Sci. 2003 Oct;19(10);522-4.
3. Benson WE, Shields JA, Tasman W, Crandall A. Posterior scleritis. A cause of diagnosis confusion.
Arch Ophthalmol.
1979 Aug;97(8):1482-6.
4. Kah TA, Premsenthil M. Posterior scleritis mimicking indirect carotid-cavernous fistula. Int J Ophthalmol Vis Sci. 2009;7:1.
5. Watson PG, Hayreh SS. Scleritis and episleritis. Br J Ophthalmol. 1976;60:163-91.
6. McCluskey PJ, Watson PG, et al. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology. 1999 Dec;106(12):2380-6.
7.
Calthorpe CM
,
Watson PG
,
McCartney AC
. Posterior scleritis: a clinical and histological survey.
Eye (Lond).
1988;2 ( Pt 3):267-77.
8. Hage R, Jean-Charles A, Guyomarch J, et al. Nodular posterior scleritis mimicking choroidal metastasis: a report of two cases.
Clin Ophthalmol.
2011;5:877-80.
9. Rossiter-Thornton M, Rossiter-Thorton L, Ghabrial R, Azar DA. Posterior scleritis mimicking orbital cellulitis.
Med J Aust.
2010 Sep 6;193(5):305-6.
10. Wong RW, Chan A. Posterior scleritis in patient with systemic lupus erythematosus. Retinal Cases Brief Reports. 2012;4:326-31.
11. Ramanathan A, Gaur A. An atypical presentation of posterior scleritis. Int J Ophthalmol Vis Sci. 2010; 8:2.
12. Ozdek SC, Gürelik G, Hasanreisoğlu B. An atypical posterior scleritis case: A diagnostic challenge. Retina. 2001;21(4):371-3.
13. Biswas J, Mittal S. Posterior scleritis: clinical profile and imaging characteristics.
Indian J Ophthalmol
.
1998 Dec;46(4):195-202.
14. Sainz de la Maza Maite. Scleritis Clinical Presentation. Medscape. Available at:
http://emedicine.medscape.com/article/1228324-clinical#showall
. Accessed October 22, 2014.
15. Auer C, Herbort C. Indocyanine green angiographic features in posterior scleritis. Am J Ophthalmol. 1998 Sep;126(3):471-6.
16. Giuffre G, Distefano M. Optical coherence tomography of chorioretinal choroidal folds.
Acta Ophthalmol Scand.
2007 May;85(3):333-6.
17. Erdol H, Kola M, Turk A. Optical coherence tomography findings in a child with posterior Eur J Ophthalmol. 2008 Nov-Dec;18(6):1007-10.
18. Sridharan S, Juneja R, Hussain A, Biswas J. Giant nodular posterior scleritis mimicking choroidal tumor. Retinal Cases Brief Reports. 2007;1:2.
19. Demirci H, Shields C, Honavar S, Shields J. Long-term follow-up of giant nodular posterior scleritis simulating choroidal melanoma.
Arch Ophthalmol.
2000 Sep;118(9):1290-2.
20. Gupta A, Gupta V, Pandav SS, Gupta A. Posterior scleritis associated with systemic tuberculosis. Indian J Ophthalmol. 2003 Dec;51(4):347-9.
21. Ikeda N, Ikeda T, Nomura C, Mimura O. Ciliochoroidal effusion syndrome associated posterior scleritis.
Jpn J Ophthalmol.
2007 Jan-Feb;51(1):49-52.
| Retina Quiz Answers: 1) a; 2) d; 3) b; 4) d; 5) c. |

