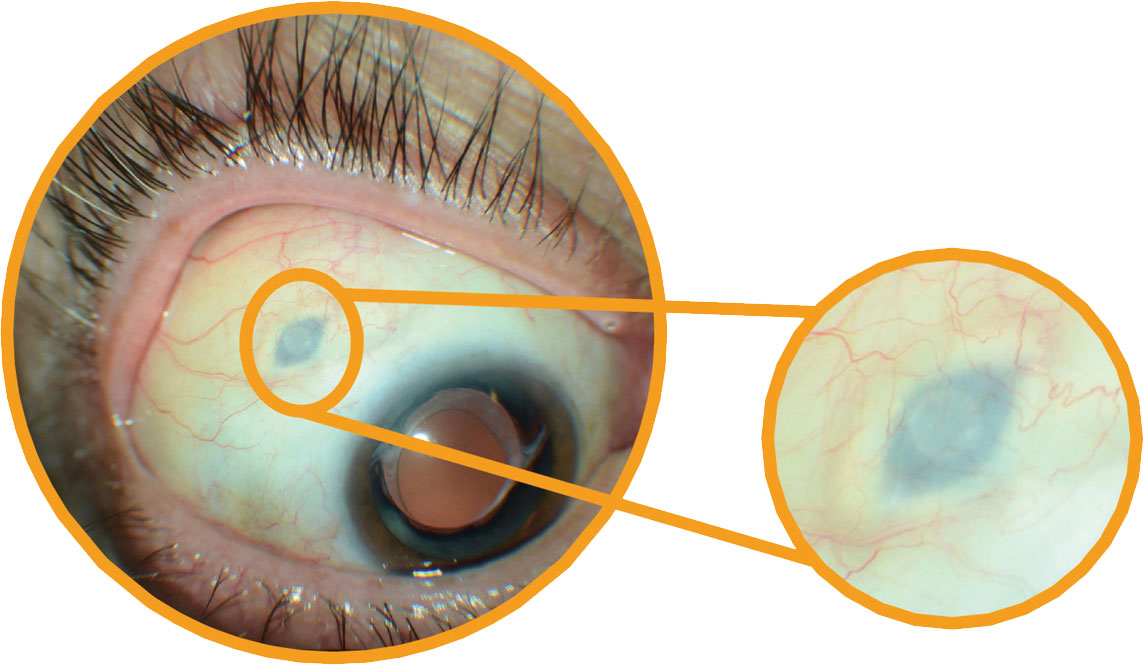
 |
| The tip of the implant remains visible beneath the conjunctiva. Click image to enlarge. |
While the average patient with wet age-related macular degeneration (AMD) today is treated via monthly or bimonthly anti-VEGF injections, a new therapeutic approach—using a refillable ocular implant that releases ranibizumab continuously—may reduce the number of yearly treatments from 12 to two. Susvimo, developed by Genentech and previously referred to as the Port Delivery System, was approved today by the FDA after demonstrating in clinical trials its ability to produce results comparable to that of monthly anti-VEGF injections.
The device is implanted into the patient’s eye during a one-time surgical procedure and must be refilled by the clinician every six months; after implantation, the implant delivers 100mg/mL ranibizumab into the eye, a press release from Genentech explains. The company notes that, if needed, supplemental ranibizumab treatment can be given while the implant is in place. During clinical trials, 98% of patients treated with Susvimo were shown to achieve and maintain vision gains at the same rate as those receiving monthly injections (+0.2 and +0.5 eye chart letters from baseline), the company reports.
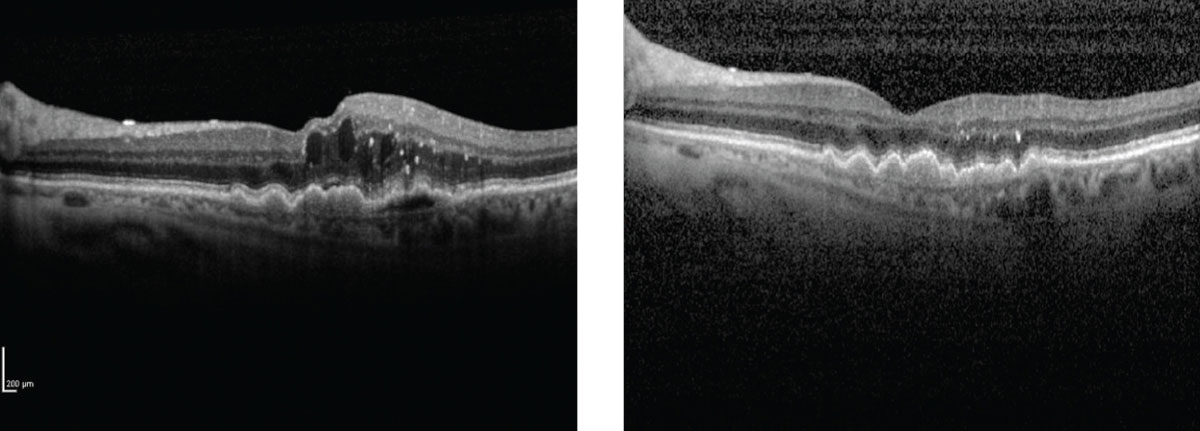 |
| Image shows a wet AMD patient before and after treatment administered by the device. Click image to enlarge. |
The most frequent complication observed was endophthalmitis, which occurred at three times the rate for patients with the Susvimo implant (2% total) than those receiving monthly ranibizumab injections. Genentech advises that close monitoring and early detection with surgical repair of conjunctival retractions or erosions may reduce the risk of endophthalmitis.
The company expects that the implant will become available throughout the United States in the coming months. This modern treatment option for wet AMD may help to reduce the burden and inaccessibility of monthly injections for many patients.
For more information, visit www.gene.com.


