When Henry Ford introduced the Model-T, he made the tongue-in-cheek comment, “It comes in any color you want, as long as you want black.” Refractive surgery began that way too, with radial keratotomy (RK) offering just one option to patients wishing to free themselves of corrective lenses. If you weren’t a good candidate, you didn’t get it, plain and simple.
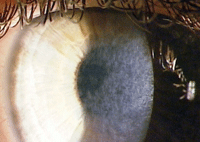 | |
| Corneal haze after PRK treatment of high myopia. |
Nowadays, so many options are available in refractive surgery it can be tough to decide which to recommend for each patient.
Excimer laser technology has given us photorefractive keratectomy (PRK) and subsequently LASIK (laser in situ keratomileusis). The excimer brought predictability and safety to refractive surgery outcomes.
Meanwhile, boundaries between cataract and refractive surgery are collapsing. Specialty intraocular lenses have brought refractive considerations to cataract surgery, while phakic IOLs and clear lens extraction allow surgeons to employ cataract techniques and technology for purely refractive purposes. More recently, femtosecond laser technology is now also used in some cataract procedures. It’s also being studied for femto-only refractive surgical procedures that could do away with the excimer altogether.
With all these changes, it’s easy to understand why recommendations are no longer so clear cut. To help bring some clarity, this article discusses three prominent controversies in choosing refractive surgery procedures: What’s the best surgical option when correcting high myopia? Which is better—monovision or multifocal IOLs? And, who really needs femto laser cataract surgery?
1. What’s the Best Refractive Surgical Option for High Myopia?
For some patients, it’s an easy decision. The 18-year-old who wears a -15.00D contact lens is best suited for a phakic IOL. The 25-year-old with a spectacle correction of -6.00-3.00x180 and a steep and thick cornea is a better candidate for LASIK or PRK.
But what do you suggest for the 40-year-old with refraction of -7.00-1.25x90, a keratometry reading of 44D and pachymetry measurement of 550µm? The refractive surgical options for this patient include PRK/LASIK, clear lens extraction or phakic IOLs. Let’s consider each.
• PRK/LASIK. In the late ’90s, the myopic treatment limits for PRK and LASIK kept expanding, which increased the pool of patients who we would accept as “good candidates.” With time, however, we realized that certain parameters need to be met to ensure great vision and a safe outcome. For example, one laser is currently approved for treatment up to -11.00D, but it’s an uncommon patient (if any) who can safely undergo this amount of corneal flattening and thinning.
Thinning the cornea too much increases the risk of ectasia, one of the most severe complications to consider with LASIK or PRK in patients with high myopia. When the cornea is too thin, the result is a weakened structure that can flex or bend in an irregular fashion. The effect is a keratoconus-like scenario that’s correctable with only a rigid lens or a corneal transplant—not a good outcome for an elective procedure!
|
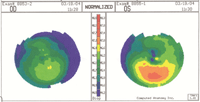
What about irregular astigmatism? Does it prevent a patient from receiving LASIK?
asymmetric astigmatism can be accounted for in refractive surgery, provided that the posterior corneal surface is normal and the corneal thickness is average. While astigmatism is a contraindication with LASIK, this scenario can still be treated—but only with PRK and only if the asymmetry is stable and not more than a couple of diopters.1
Also, if the posterior cornea is bowed inward or doesn’t parallel the contour of the
So, first determine whether the posterior corneal surface is normal. Advanced topography instruments (e.g., Pentacam, Galilei, Orbscan) measure both the anterior and posterior corneal curvatures; the posterior corneal curvature is called posterior float. Usually, patients with an abnormal posterior float should not have laser refractive surgery. But if the corneal thickness is at least average and doesn’t thin over the area of the abnormal float, the Rx is mild to moderate (less than 3D) and the posterior float is in the normal range, then this patient is a possible candidate for PRK. |
Most seasoned clinicians now prefer a residual stromal bed of 300µm to 350µm left in the cornea. How do you calculate this? First, determine how much tissue will be ablated. To do so, multiply the spherical equivalent of the refractive error by 15µm, a ballpark number of how much tissue is ablated per diopter. For example: a -6D myope will lose 90µm (6 x 15) of central corneal thickness on average. Now that you can gauge the extent of corneal thinning, and take into account that the typical flap is about 100µm, then add up these numbers along with the desired residual thickness. You’ll find that the -6D patient requires a central corneal thickness of at least 540µm to be safe (100 + 90 + 350 = 540).
Another guideline that many practices try to follow is to limit laser ablation of the corneal stroma to 100µm or less, if at all possible. So, if the math cuts it too close, choose PRK. Remember, because no flap is created with PRK, you retain an extra 100µm of corneal stroma. Although the epithelium is removed at the time of PRK surgery, it grows back in about three days with the assistance of a bandage soft contact lens.
The other number to consider is the keratometry (K) reading, the measurement of the curvature of the anterior surface of the cornea. Quality of vision, which really relates to contrast sensitivity, relies heavily on the resultant K reading. Ideally, the patient’s resultant K should be 39D or steeper to preserve quality of vision. When flattening the center beyond this, corneal higher-order aberrations increase and you lose the natural prolate corneal anatomy. If treating a hyperope, post-op Ks should be under 50, as a rule of thumb.
To determine the post-op K reading using the initial K reading, take the spherical equivalent of the myopic spectacle correction and multiply by 0.7 (a shorthand number to determine the corneal power). For example: 8D x 0.7 yields 5.6. If the patient’s initial K reading is steep—say, 45D or 46D—then this patient would be in the group expected to do best (45 - 5.6 = 39.4).
PRK is an option for higher corrections to avoid thinning the cornea too much. (Remember, you retain 100µm of untouched stroma.) However, when treating high myopia, a significant incidence of corneal haze and loss of best-corrected acuity can occur (with or without mitomycin). To decrease this chance of corneal haze and scarring, it is best to keep the amount of laser treatment under 100µm regardless of the corneal thickness.
The third finding to consider is the pupillary diameter. If the scotopic pupil is large (greater than 7mm), the ablation zone diameter must be enlarged to lessen or avoid the halo effect with night vision. However, if the treatment diameter is enlarged, the 15µm per diopter rule no longer applies; it could be more like 20µm/D.
• Clear lens extraction or phakic IOLs. These options spare the cornea and avoid the potential for ectasia (since no stroma is removed) and neurotrophic keratitis (as corneal sensitivity is unchanged). But these procedures are surgically more complex and more expensive than LASIK or PRK, carry a risk of intraocular infection and are usually performed one eye at a time.
Clear lens extraction (CLE) replaces a non-cataractous lens with an IOL, while phakic IOLs preserve the existing crystalline lens and add a little more refractive horsepower with a lens implant. Phakic IOLs can be positioned either anterior to the iris (the Veriseye, Abbott Medical Optics) or posterior to it (the Visian ICL, Staar Surgical). Phakic IOLs offer the advantages of reversibility and the preservation of accommodation, and the Visian ICL eliminates concerns about halo because the optics are behind the pupil. When compared to high myopic LASIK, outcomes with the ICL are said to offer better quality of vision.2
The Visian ICL is usually chosen for individuals who are younger and not presbyopic—it’s FDA approved for ages 21 to 45—or not a candidate for corneal refractive surgery. The device is not currently available in toric powers in the United States, so sometimes the ICL has to be combined with LASIK or PRK to correct astigmatism.
Probably the biggest surgical concern for the Visian ICL is touching the crystalline lens and inducing an anterior cortical or subcapsular cataract. The major disadvantage of the Veriseye is that it is physically attached to the iris, which can cause pigmentary dispersion and chronic inflammation.
The ICL implant requires an anterior chamber depth of 3.0mm or greater, which is usually not an issue in most high myopes. One or two YAG laser peripheral iridotomies are performed one week preoperatively to prevent pupillary block glaucoma.
Clear lens extraction is less expensive than ICL, less surgically challenging and offers the advantage of correcting astigmatism with a toric implant. It is the modality chosen for an older presbyopic patient. Clear lens extraction can also be performed on hyperopes, whereas the Visian only corrects -3D to -20D of myopia. Besides, hyperopes would be poorly suited to the ICL because they tend to have shallow anterior chambers, increasing the risk of pupillary block/angle closure glaucoma.
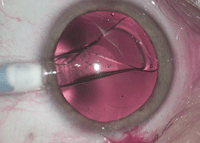 | |
| For high myopia, an implantable collamer lens can offer better quality of vision than LASIK. |
In summary, the older presbyopic patient with a normal peripheral retina would do best with clear lens extraction. The younger patient with less than 7D of myopia who has a normal corneal pachymetry (550µm or thicker) and typical corneal steepness is generally a LASIK or PRK candidate. The patient who is not presbyopic with this same correction but with large pupils or with collagen vascular disease and dry eyes would probably be best served with the ICL.
So, in our 40-year-old patient, if he has no retinal issues and no sign of early cataract, I’d suggest LASIK, or an ICL if the scotopic pupil is 7mm or if there is any collagen vascular disease or dry eye.
2. Which Patients Are Best Suited to Multifocal IOL, Accommodative IOL or Blended Vision/Monovision?
It’s difficult to predict which patients will be able to tolerate a multifocal. A multifocal IOL is analogous to a multifocal soft contact lens in the visual compromise and adaptation period needed, but with one huge difference: it’s not so easy to remove an IOL. Optometrists have come to expect that some patients will have visual concerns after this procedure, as they often do with multifocal contacts.
Simply put, multifocals are great—for some people; it’s just challenging to tell which ones. Of course, we all want our patients to be “100% happy,” but in my experience, too many poorly selected patients complain of compromised vision, night-time obscurations and dysphotopsias (unusual temporal shadows) with multifocal IOLs. At least one study found a higher rate of patient satisfaction with monovision, without the risk of visual obscurations and the added cost that multifocal IOLs incur.3
Hyperopes seem to have the highest acceptance rate for multifocal IOLs, but they also do extremely well with monovision. Depth of focus, which is a function of a smaller pupillary diameter, seems to work best with hyperopic patients, and some are able to read and see in the distance with a standard monofocal IOL.
Blended vision or modified monovision also works well.3 With a residual refractive error of -1.25D to -1.50D in the non-dominant eye, it’s amazing how well these patients function in good illumination. If your patient has been successful in the past with monovision with contact lenses or LASIK, they almost always do well with this strategy using IOLs.
The accommodating or flexing IOLs have fewer dysphotopsias compared to multifocal IOLs, but sometimes experience flexure anomalies that compromise the patient’s final refractive result. They also have a smaller optic zone compared to standard monofocal IOLs, which can negatively affect night vision. Also, compared to the newer monofocal IOL designs, they may have a higher incidence of posterior capsular opacity.4
3. Who Really Needs Femto Laser Cataract Surgery?
Surgeons disagree on this question, with some advocating routine use of femto, others believing its greater precision is primarily a benefit only to those patients receiving a premium IOL, and still others waiting for more compelling safety and outcomes data to show up in the literature before adopting the technology.
As with any other laser procedure, results are more predictable than with manual techniques. The femto laser makes a perfect corneal incision and, more importantly, anterior capsulorhexis. That is often the most challenging and important part of the surgery regarding post-op IOL performance. This is especially advantageous in patients who opt for premium IOLs. When the capsulorhexis is perfectly sized and centered, toric and multifocal IOLs have the best chance of exact centration and least chance of tilt.
 |
|
|
A femtosecond laser neatly fragments the lens nucleus, but even better is its ability to make a perfectly circular capsulorhexis.
Photo: Omni Eye Services of Atlanta.
|
Other patients who are more likely to benefit from use of femto technology are those with a dense brunescent nuclear sclerosis, low endothelial cell count or hypermature cataract (a totally white cataract makes it more difficult to perform the capsulorhexis). Also, the patient with 1.5D or less of corneal astigmatism usually benefits from the precision of the femto laser’s limbal relaxing incisions; above that range, a toric IOL may offer a better solution.
Still, skilled high-volume surgeons can often perform just as well with handheld instruments. Younger and less experienced surgeons may come to rely on the femto more than veterans. (Perhaps ironically, the best surgeons are the ones investing in this expensive technology, but most likely need it the least.)
As this element of the surgery is a non-covered benefit, patients have a higher out-of-pocket fee when the femto laser is used. Patients have to weigh the purported benefits against the added costs; some certainly do gravitate toward the newest techniques and the latest technology, but not all senior citizens on a fixed income will.
Traumatic cataracts that often have zonular dehiscence should not undergo a femto procedure; the laser fragmentation can result in a sudden loss of the nucleus into the vitreous. Patients with a history of anterior basement membrane disease or recurrent corneal erosion are not good candidates either, as the laser docking can cause a corneal abrasion, depending on the laser’s interface. Small orbital fissures can complicate the procedure and patients with prior RK or penetrating keratoplasty should avoid it. It’s also contraindicated for LASIK patients, as gas bubbles can go under the flap.
In summary, we like the femto technology and offer it to patients who can benefit from the laser limbal relaxing incisions, have hard brunescent nuclei or significant amount of corneal endothelial issues. Time will tell if this marvelous technology makes enough of a difference in surgical outcomes (complications and final vision) compared to standard phacoemulsification to become standard of care.
Dr. Den Beste is the founder of Lasik Pro Eye Consultants in Orlando, Fla.
1. Alpins N, Stamatelatos G. Customized photoastigmatic refractive keratectomy using combined topographic and refractive data for myopia and astigmatism in eyes with forme fruste and mild keratoconus. J Cataract Refract Surg.2007; 33(4):591-602.
2. Sanders DR. Matched population comparison of the Visian Implantable Collamer Lens and standard LASIK for myopia of -3.00 to -7.88 diopters. J Refract Surg. 2007 Jun; 23(6):537-53.
3. Zhang F, Sugar A, Jacobsen G, Collins M. Visual function and patient satisfaction: Comparison between bilateral diffractive multifocal intraocular lenses and monovision pseudophakia. J Cataract Refract Surg. 2011 Mar;37(3):446-53.
4. Takakura A, Iyer P, Adams JR, Pepin SM. Functional assessment of accommodating intraocular lenses versus monofocal intraocular lenses in cataract surgery: metaanalysis. J Cataract Refract Surg. 2010 Mar;36(3):380-8.