 |
A 75-year-old African-American female presented to the office acutely with a chief complaint of ocular pain and redness OS of four days’ duration. Her ocular history was remarkable for uneventful phacoemulsification surgery in that eye, completed two weeks prior. Systemic history was positive for hypertension, for which she was properly medicated and compliant. The patient denied allergies of any kind.
Clinical Findings
Her best-corrected entering visual acuities were 20/20 OD and 20/400 OS, with no improvement using pinhole at distance or near. Her external examination was normal. There was excessive lacrimation with conjunctival injection OS. There was no evidence of afferent pupillary defect. The pertinent biomicroscopic examination of the anterior segment OS is demonstrated in the photographs,. Goldmann applanation tonometry measured 15mm Hg OD and 65mm Hg OS.
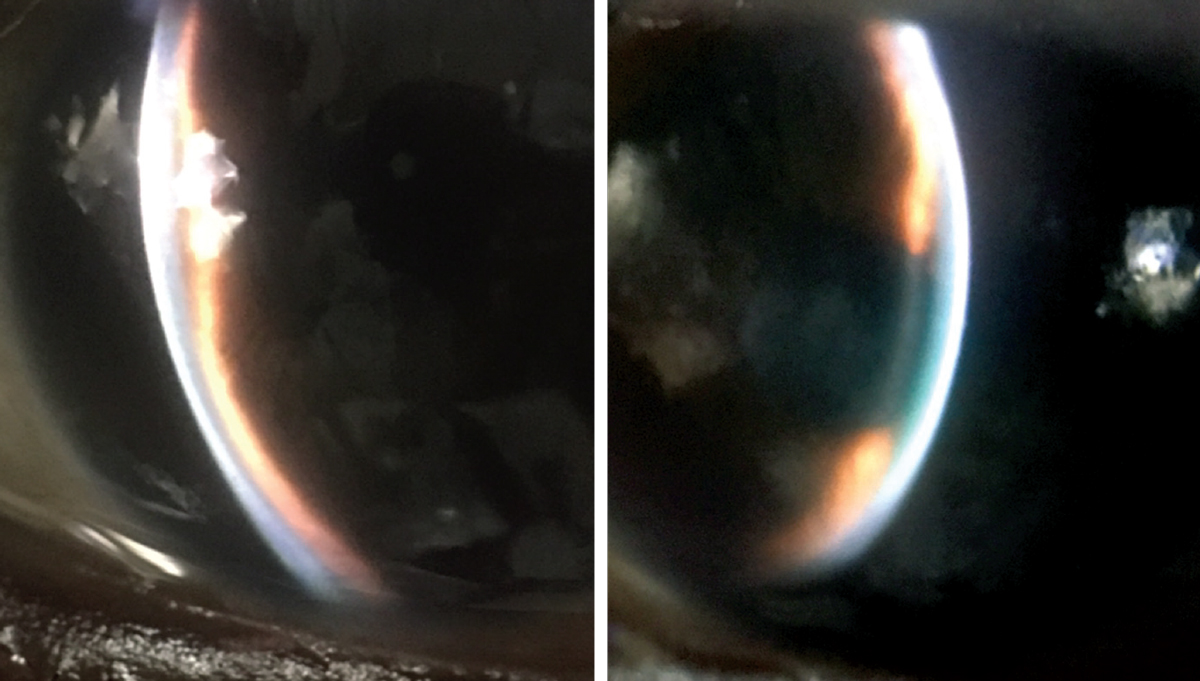 |
Biomicroscopy and ultrasound biomicroscopy yielded the findings in the images above and below. What do they—and the case history—point toward? Click to enlarge. |
For More Information
Additional studies might have included gonioscopy, B-scan ultrasonography, anterior segment and angle optical coherence tomography and ultrasound biomicroscopy (obtained in this case and shown below).
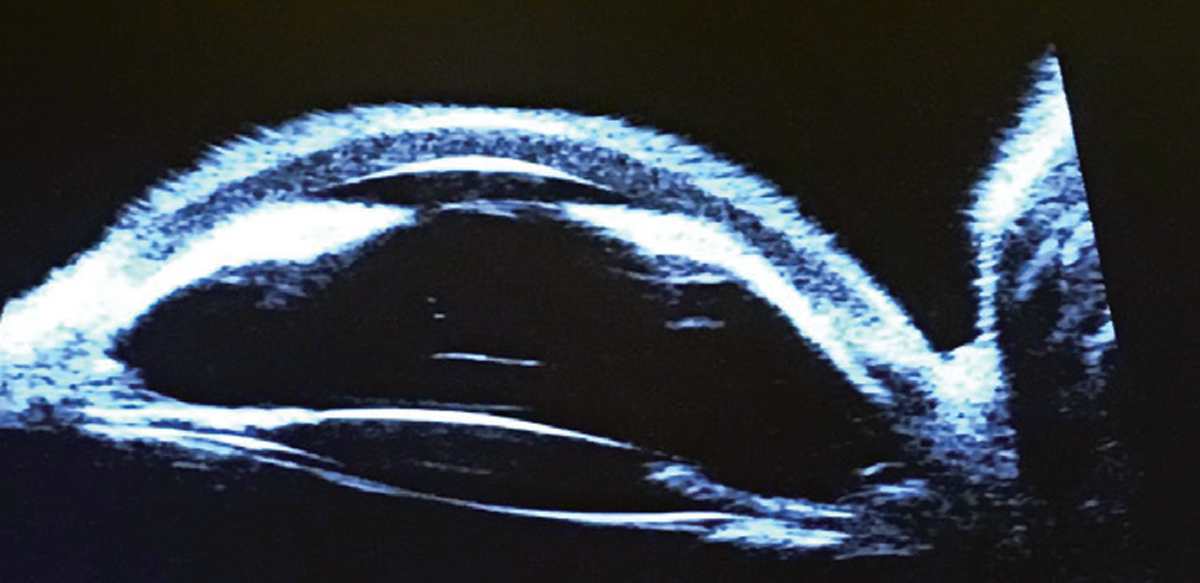 |
| Click image to enlarge. |
Additionally, history questions should be asked regarding inadvertent trauma, which may have produced accidental hypotony with or without a cyclodialysis cleft. More questions might include use of any medication from the sulfonamide group (e.g., oral acetazolamide, topiramate), which may cause an idiosyncratic uveal effusion with forward movement of both the choroid and ciliary body.
Immediate Care
First aid for lowering the elevated intraocular pressure (IOP) included the installation of one drop each of topical iopidine 1%, timoptic 0.5%, travoprost and brinzolamide. The oral anti-glaucoma medications acetazolamide and methazolamide were not used. Given the number and presence of Peripheral anterior synechiae (PAS), atropine therapy was not attempted. A topical steroid was used QID to decrease inflammation. The IOP was reduced in the office with this treatment to 22mm Hg in 60 minutes’ time.
Diagnosis
This patient is suffering from secondary angle closure with post-surgical fibrin-related pupil block, causing a resultant complete pupillary seclusion (blockage) and angle obliteration.
The diagnosis was derived by careful clinical observation of the anterior segment and its structures along with interpretation of the ultrasound biomicroscopy result.
The four principal differential diagnoses in this case were:1-17
- Soemmering’s ring pupil block angle closure glaucoma.
- Aqueous misdirection syndrome (malignant glaucoma, IOL displacement with pupil block).
- Uveitic glaucoma with secondary angle closure.
- Choroidal effusion with “choroidal” formations secondary to fluid seepage or suprachoroidal hemorrhage producing secondary angle closure.
These things were easily ruled out using ultrasonography instruments. Soemmering’s ring is defined as a circumferential proliferation of residual lenticular epithelial cells, not aspirated during phacoemulsification, forming a connection to the iris creating a progressive synechial pupillary block.1
The Ritch 4-point system can be used to categorize a “push” pupil block (the lens iris diaphragm is pushed forward until the angle is occluded) into four anatomical levels (iris, ciliary body, lens and structures posterior to the lens).1 In cases of where this is suspected, ultrasound biomicroscopy will demonstrate an enlarging connection at the level of the remaining lens as the cells make connection with the iris in the absence of ciliary body rotation, iridociliary cysts, iris tumor, plateau iris, dislocation or subluxation of the lens, iridovitreous connection, iridocapsular connection, malignant glaucoma or suprachoroidal hemorrhage/effusion.1
Conditions that may cause the “pull” mechanism of angle closure (the iris and corneal tissues are contracted together occluding the angle) include iris and angle neovascularization and iridocorneal endothelial syndromes.1 Exfoliation syndrome is a known agitator of Soemmering’s ring and increases the risk of potential synechial angle closure when conditions are favorable.1
Aqueous misdirection is a form of secondary angle closure glaucoma marked by elevated intraocular pressures, myopic shift and central shallowing of the anterior chamber.2-7 The term was first used by von Graefe in 1869.2,3 While it commonly occurs following phacoemulsification and filtering surgery, it can also occur after cyclophotocoagulation surgery, YAG laser capsulotomy and vitrectomy.2,7 It can occur in phakic and aphakic patients and idiopathically as well.2-8
The exact mechanism by which aqueous misdirection occurs is still poorly understood.2-7 It is hypothesized that an abnormal anatomic relationship develops between the ciliary body, vitreous and lens which causes the anterior hyaloid vitreous membrane to be in a position which favors diversion of aqueous humor backward into the posterior chamber.2-7 The resultant forces push the vitreous and its anterior hyaloid membrane forward displacing the lens–iris diaphragm.2-7 Ultrasound biomicroscopy can be used to demonstrate fluid accumulation and rotated ciliary processes.2-7
In aqueous misdirection, the anterior hyaloid/posterior lens communication facilitates the trapping of aqueous and induces pressure elevation. The increased pressure leads to forward movement of the iris and creates shallowing of the anterior chamber which is capable of becoming complete angle closure. These changes often develop immediately following surgery, however, the condition can occur many years after the after the procedure. Alternate theories include post-surgical expansion of the choroid producing increased IOP, which leads to forward displacement of the lens–iris diaphragm and a theory where aqueous pools posterior to the vitreous causing anterior displacement of vitreous body, lens, ciliary body and iris.2,4-6 Having aqueous misdirection in one eye increases the risk in the fellow eye.
Management
Treatment for aqueous misdirection includes lowering the IOP acutely with atropine 1% and any combination of oral and topical anti-glaucoma medications along with decreasing the inflammation with a topical steroidal preparation. Surgically, the condition is remedied by completing a core vitrectomy to eliminate the vitreous pupil block and then finishing with a 360° goniosynechialysis to re-form the anterior chamber.7-9 Ocular hypotensive medications can be continued for as long necessary. Occasionally, an alternative surgical solution, such as a trabeculectomy, will be required when IOP requires additional control.
The terms uveal effusion, choroidal effusion, ciliochoroidal effusion, ciliochoroidal detachment and choroidal detachment are all used to connote the same thing.10-15 They describe the process where an abnormal collection of fluid expands in the suprachoroidal space, producing internal elevation of the choroid.10-15 Choroidal detachment (CD) occurs when the choroidal layers separate from the scleral shell.10-12 It can be observed during the early post-operative period of any ocular surgery, where IOP is intentionally or accidentally lowered but the incidence is higher particularly after trabeculectomy and glaucoma drainage implant surgery.
Topical antiglaucoma agents and idiopathic cases have been recognized in the literature.10 Suprachoroidal hemorrhage is a complication of intraocular surgery that can cause CD.10,11 Spontaneous suprachoroidal hemorrhage is also recorded in the literature.11 Uveal effusion after cataract surgery is associated with postoperative ocular injury, chronic post-surgical uveitis, the presence of active vascular diseases such as Sturge-Weber syndrome and malignancy.13
The pathophysiology of CD in ocular injury, surgery, infection or inflammation is choroidal fluid transudation.10-13 Macromolecular diffusion interferes with normal transscleral egress of albumin out of the eye. This causes choroidal fluid retention and altered osmotic forces.15 An alternative hypothesis finds a swollen sclera compressing transscleral vessels with resulting fluid retention.15 This fluid induces the choroidal and scleral layers to separate.10-12
Increasing uveoscleral outflow, by any means (pharmacologic or uveitic) has the potential to reduce and alter collagen types I, III, and IV in the ciliary smooth muscles and adjacent sclera.12 This alteration has also been linked to the creation of supraciliochoroidal fluid.12 Certain topical anti-glaucoma preparations and oral sulfa derivatives have an action on the extracellular matrix of the ciliary muscle; by activation of matrix metalloproteinases processes they set in motion a favorable environment for CD formation.12,13 CD may also be produced by iatrogenic cyclodialysis due to any anterior segment injury or intraocular procedure.12 Chronic disease may lead to secondary retinal pigment epithelial (leopard spot) changes and permanently reduced visual acuity.15
In the acute phases of CD IOP can be controlled with topical and oral medications for as long as they are required. As the IOP issue resolves, anti-glaucoma agents can be systematically withdrawn. Topical prednisolone 1% can be prescribed aggressively along with topical atropine 1% BID for inflammation control. Oral prednisolone 20 mg po tid can be used to compliment the inflammation control and stabilize the transudation of fluids within the eye. It can be prescribed with or without oral ranitidine.10-13 Subscleral sclerectomy is an effective method for tapping and draining the fluid in recalcitrant cases.
Postoperative fibrin pupillary-block glaucoma is a rare complication of intraocular surgery.16,17 It develops when an inflammatory fibrin membrane occludes the pupil.16,17 The resulting increased inflammatory processes can produce combinations of peripheral anterior synechae (PAS) and posterior synechae (PS) causing both pupil block and peripheral angle closure.16,17
While the finding is largely idiopathic, hypertension and diabetes are proposed risk factors.17
The treatment for fibrin pupillary-block glaucoma includes topical and oral agents for lowering IOP along with both laser peripheral iridotomy and laser lysis of the fibrin membrane. Intraocular transforming plasminogen activating factor has also been used.16,17 Mechanical reformation of the angle is completed, breaking the synechiae and deepening the anterior chamber when necessary. The reason additional modalities must be included in the management is because when the membrane is disrupted alone the opening often self-seals secondary to residual sticky debris which permits its reformation.17 Disrupting iridocorneal adhesions lessens the risk of chronic corneal edema and endothelial cell damage.17 Topical steroids can be used to manage inflammation along with atropine 1% as required.
In addition to the in-office first-aid, this patient was treated with topical ocular hypotensives, lowering the pressure into the 20s until they could be seen by the operating surgeon where they were emergently referred. They underwent YAG lysis of the fibrin membrane on the same day. The next day they were taken to the operating room, where the anterior chamber was mechanically restored and the remnants of the membrane removed.
The IOP recovered to the high teens on the day of the procedure and settled in at nine within two days. The visual acuity returned as well. All topical ocular hypotensives were removed over the course of a week.
Dr. Gurwood thanks Dr. J. Lewis, MD and P. McManamon OD for contributing this case.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
1. Kung Y, Park SC, Liebmann JM, Ritch R. Progressive synechial angle closure from an enlarging Soemmering ring. Arch Ophthalmol. 2011;129(12):1631-2. 2. Moinul P, Hutnik CM. Aqueous misdirection syndrome: an interesting case presentation. Clin Ophthalmol. 2015;9:183-6. 3. Von Graefe A. Bietrage zur Pathologie, Therapie de Glaucoma. Arch ophthalmol 1869;15:108. 4. Quigley HA. Angle-closure glaucoma-simpler answers to complex mechanisms: LXVI Edward Jackson memorial lecture. Am J Ophthalmol. 2009;148(5):657–669. 5. Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12(2):167–180. 6. Shaffer RN, Hoskins HD., Jr Ciliary block (malignant) glaucoma. Ophthalmology. 1978; 85(3):215–221. 7. Parivadhini A, Lingam V. Management of Secondary Angle Closure Glaucoma. J Curr Glaucoma Pract. 2014;8(1):25-32. 8. Jarade EF, Dirani A, Jabbour E, et al. Spontaneous simultaneous bilateral malignant glaucoma of a patient with no antecedent history of medical or surgical eye diseases. Clin Ophthalmol. 2014 ;8:1047-50. 9. Dervenis N, Mikropoulou AM, Dervenis P, Lewis A. Anterior segment approach for the surgical management of aqueous misdirection syndrome. J Cataract Refract Surg. 2017;43(7):877-878. 10. Xu H, Lutrin D, Wu Z. Outcomes of 23-gauge pars plana vitrectomy combined with phacoemulsification and capsulotomy without intraocular lens implantation in rhegmatogenous retinal detachment associated with choroidal detachment. Medicine (Baltimore). 2017;96(34):e7869. 11. Nakakura S, Noguchi A, Tabuchi H, Kiuchi Y. Bimatoprost-induced late-onset choroidal detachment after trabeculectomy: A case report and review of the literature. Medicine (Baltimore). 2017;96(5):e5927. 12. Saluja K, Naik M, Vemparala R, Mehta A. Idiopathic Bilateral Suprachoroidal Haemorrhage: A Rare Case Presentation. Case Rep Ophthalmol Med. 2017;2017:4234238. 13. Krishnamurthy R, Senthil S, Garudadri CS. Late postoperative choroidal detachment following an uneventful cataract surgery in a patient on topical latanoprost. BMJ Case Rep. 2015;2015. pii: bcr2015211408. 14. Uyama M, Takahashi K, Kozaki J, et al. Uveal effusion syndrome: clinical features, surgical treatment, histologic examination of the sclera, and pathophysiology. Ophthalmology. 2000;107(3):441-9. 15. Elagouz M, Stanescu-Segall D, Jackson TL. Uveal effusion syndrome. Surv Ophthalmol. 2010;55(2):134-45. 16. Khor WB, Perera S, Jap A, et al. Anterior segment imaging in the management of postoperative fibrin pupillary-block glaucoma. J Cataract Refract Surg. 2009;35(7):1307-12. 17. Yoshino H, Seki M, Ueda J, et al. Fibrin membrane pupillary-block glaucoma after uneventful cataract surgery treated with intracameral tissue plasminogen activator: a case report. BMC Ophthalmol. 2012;12:3. |

