 |
Uveal effusion syndrome (UES) is a rare clinical entity with the potential to cause devastating visual consequences. Uveal effusion itself refers to transudation from the choriocapillaris into the suprachoroidal space, which causes subsequent choroidal thickening and detachment.1 It may also lead to subretinal fluid and serous retinal detachments.2 Causes of uveal effusion include inflammation—which can result from trauma, scleritis, photocoagulation or uveitis—and hydrostatic conditions such as hypotony, primary angle closure glaucoma and drug-induced, arterio-venous fistula.1
When uveal effusion occurs in the absence of inflammation or hydrostatic changes, the term UES applies, making it a diagnosis of exclusion.1–3 Investigators believe UES patients have an abnormality of the choroid or sclera.1,4 Most cases of UES involve peripheral choroidal detachments. However, posterior uveal effusion syndrome (PUES), a variant of UES, remains isolated to the posterior pole.4,5
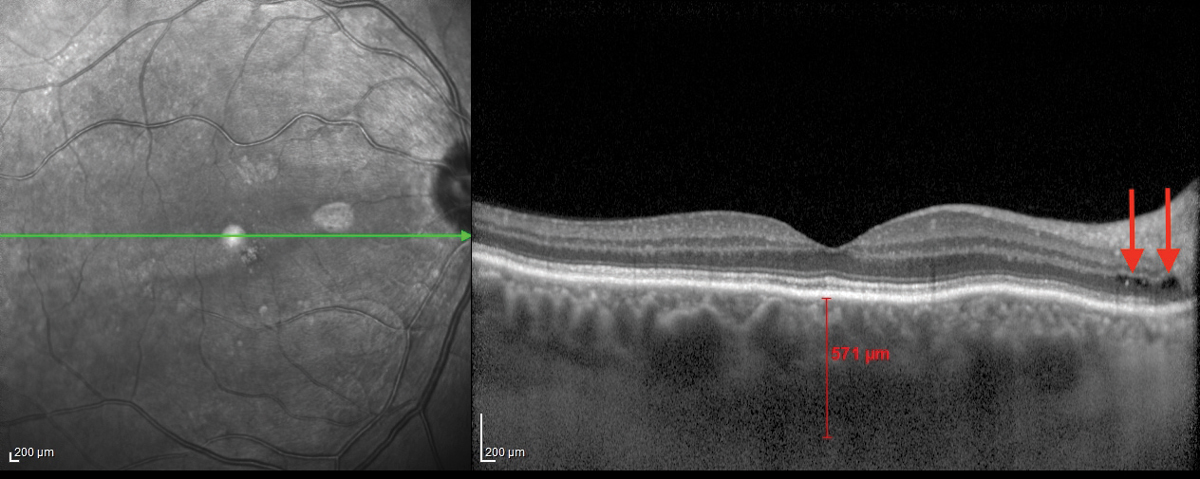 |
| Fig. 1. This OCT B-scan shows the patient’s right eye. Click image to enlarge. |
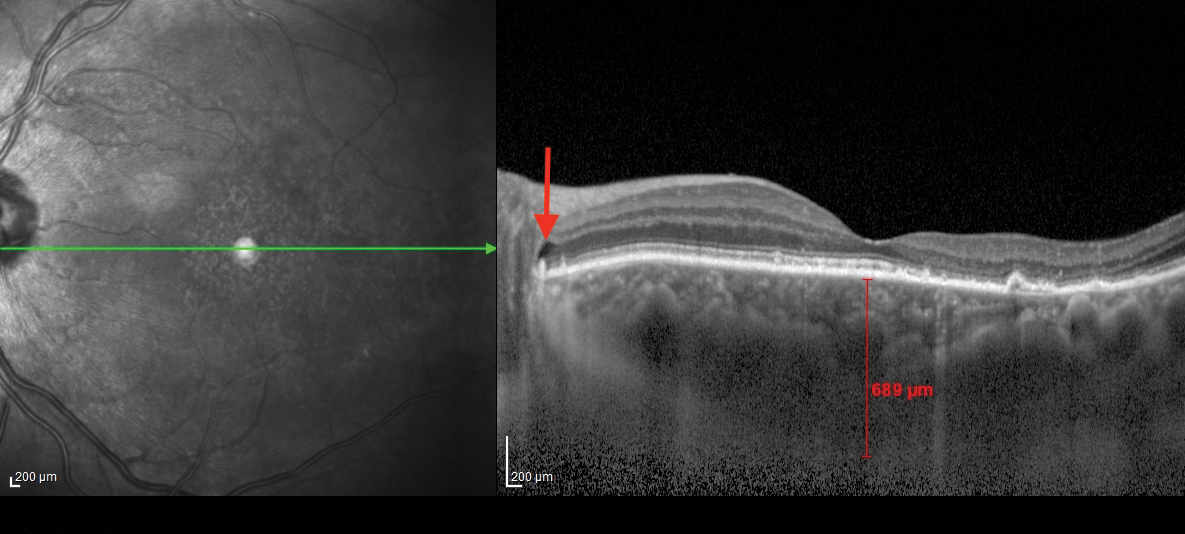 |
| Fig. 2. This OCT B-scan shows the patient’s left eye.The posterior subretinal fluid that may have extended from the cystic spaces is noted with red arrows at the optic disc margin. Click image to enlarge. |
Case Example
A 59-year-old Caucasian male presented with a chief complaint of blurred spots in his vision, more so in his left eye than his right. The spots were constant and did not change with eye movement. The symptoms in his left eye had been present since he underwent cataract surgery in 2012 with gradual enlargement and changes in shape of the spots. Conversely, the area in his right eye had only been present for two weeks. The patient described his vision as “looking through water.”
His best-corrected visual acuity measured 20/20 OD with +1.50D, and 20/30+2 OS with +0.75-0.75x090. His ocular history included pterygium removal from the left eye in 1980. His medical history was positive for hypertension and gout. No medication use was reported. Anterior segment examination revealed nasal corneal scarring consistent with the previous pterygium surgery in the left eye; and presbyopia-correcting intraocular lenses in both eyes with mild posterior capsular opacification. Prior to cataract development, the patient maintained acuities of 20/20 OD and OS with a refractive error of +3.00 OD, and +3.75 OS. Intraocular pressures (IOP) obtained with Goldmann tonometry were 13mm Hg OD and OS. Dilated fundus exam results included flat, pink optic nerves with a cup-to-disc ratio of 0.25 round OU. Scattered areas of retinal pigment epithelial (RPE) disruption were noted within the posterior pole in both eyes. Optical coherence tomography B-scans (OCT-B) with enhanced depth imaging (EDI) were obtained along with fundus autofluorescence (FAF) pictures and ultrasound B-scans.
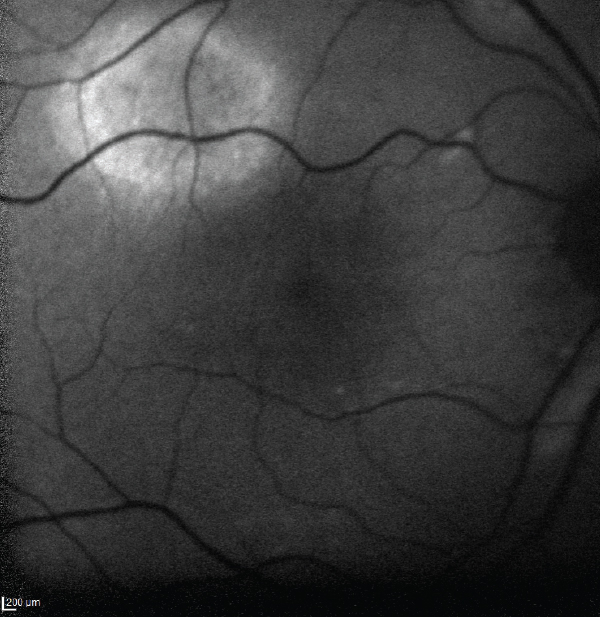 |  |
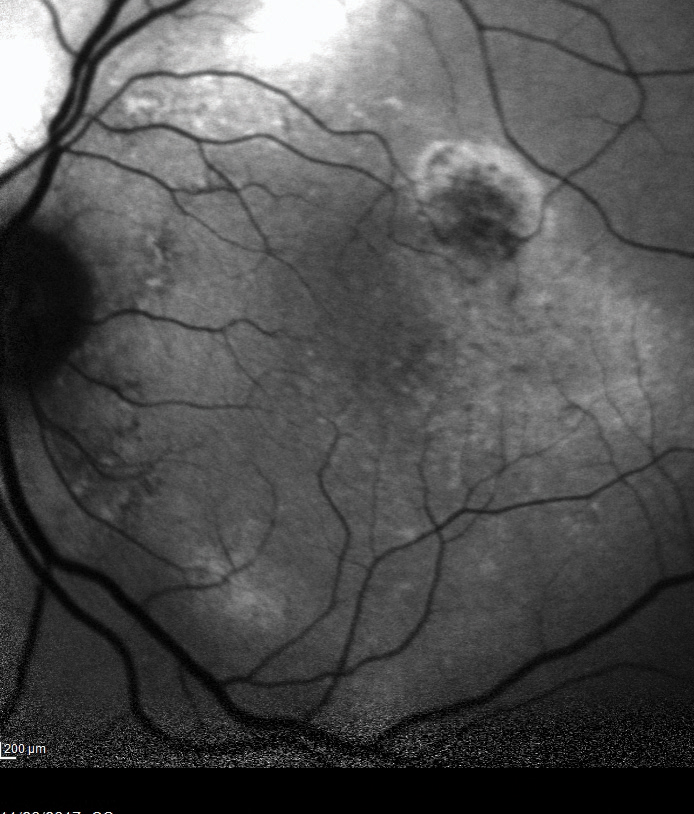
| Fig. 3. This image shows an area of autofluorescence in the superotemporal arcade of the right eye. | Fig. 4. OCT-B scans in the same area reveal a serous retinal detachment. |
The OCT-B EDI scans (Figures 1 and 2) demonstrated marked pachychoroid of nearly 600µm OD and 700µm OS. FAF of the right eye highlighted an area of autofluroescence approximately the size of the macula inside the superior temporal arcade (Figure 3). OCT-B scans of this area confirmed a serous retinal detachment (Figure 4). The more diffuse autofluorescence in the FAF image of the left eye (Figure 5) included two areas of increased intensity superior and superotemporal to the optic nerve (exaggerated due to instrument gain amplification). A retinal thickness map of the segmented outer nuclear layer (ONL) in the left eye (Figure 6) illustrated the extensive areas of ONL atrophy. The “hot spot” correlated to a focal retinoschisis. The FAF of the left eye also shows an area absent of autofluorescence about 1DD superotemporal to the macula. An OCT-B scan through this area revealed outer retina and RPE atrophy (Figure 7). The ultrasound B-scan (Figure 8) exhibited a thickened retina-choroid-sclera complex, but no peripheral choroidal detachments, which correlated to clinical findings. An ultrasound biomicroscopy scan was not obtained due to the lack of peripheral choroidal involvement.
Discussion
UES is an uncommon diagnosis where uveal effusion and serous retinal detachment are the most characteristic clinical findings.1 It has a middle aged-male predilection, and 65% of cases occur bilaterally.1,3 UES is closely related to nanophthalmos or hyperopia and a thick sclera, and is rarely accompanied by significant inflammation.1,6 It results from abnormal transscleral diffusion of choroidal extravascular proteins from the suprachoroidal space.3,7 This leads to vortex vein compression and subsequent congestion of choroidal veins.3 Pachychoroid ensues from fluid accumulation, thus setting the stage for ciliochoroidal and non-rhegmatogenous retinal detachments.
When UES is confined to the posterior pole, the alternative diagnosis PUES applies. Speculation exists as to the mechanism of fluid transfer into the posterior pole. In this patient, the posterior subretinal fluid may have extended from the cystic spaces noted at the optic disc margin—see the arrows in Figures 1 and 2. Research shows this in patients with PUES.4,5 One team even postulated that a combination of RPE and photoreceptor loss in the presence of a temporal disc crescent could facilitate the movement of choroidal fluid to the neurosensory retina.5 This anatomical appearance of a thick peripapillary choroid and disc crescent may present a weak link in the blood-retinal barrier.4 A fluorescein angiogram could pinpoint these areas of peripapillary leakage (this patient declined consent for the procedure).
UES is thought to be extremely rare and few reports of PUES exist in the literature.1,3-5 This may be due to a lack of diagnostic acumen. Middle-aged to older patients presenting with pigmentary changes and subretinal fluid may erroneously be diagnosed with exudative macular degeneration. In these cases, the clinician should image the choroid with OCT-B EDI to evaluate for pachychoroid.
 | 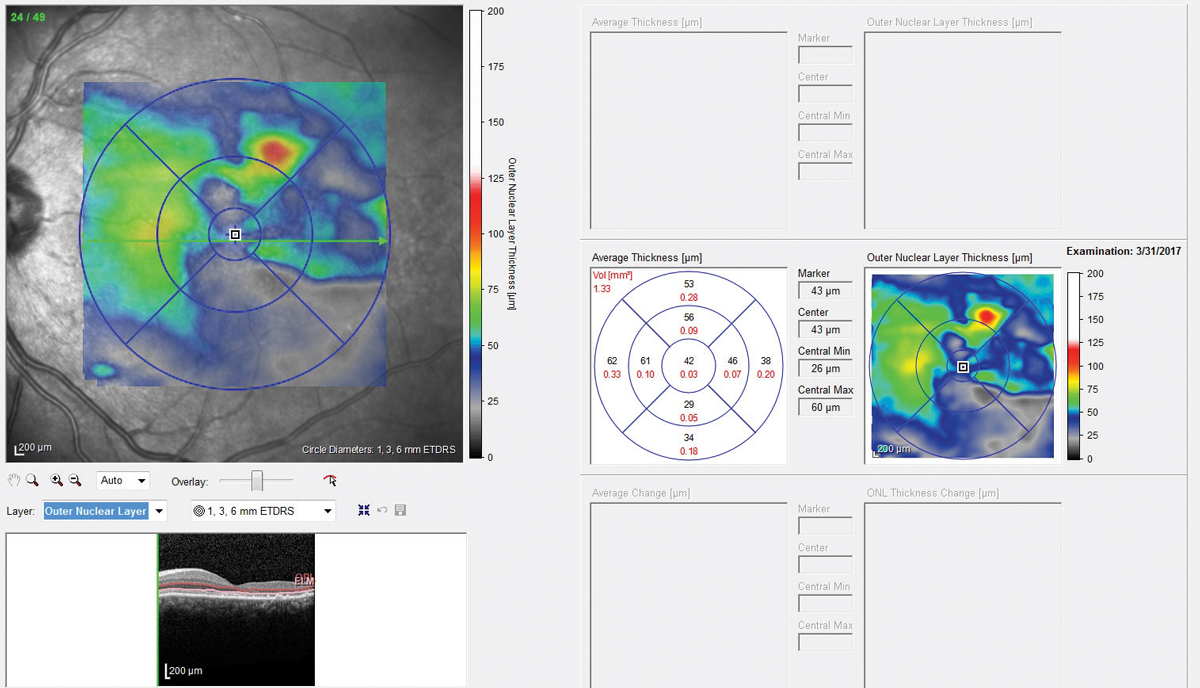 |
| Fig. 5. Fundus autofluorescence OS shows two areas of increased intensity superior and superotemporal to the optic nerve. | Fig. 6. A retinal thickness map of the eye illustrated extensive areas of outer nuclear layer atrophy. Click image to enlarge. |
PUES Diagnoses
The disease may also be confused with central serous chorioretinopathy (CSR) as similar clinical findings, multimodal imaging results and demographics (male predilection) exist. As with this patient, comparable data made CSR a strong diagnostic contender. The lean toward an ultimate PUES diagnosis hinged on several factors.
First, a meta-analysis found the mean subfoveal choroidal thickness in patients affected by CSR to be 413.1 ± 93.0µm.8 Using this data, the right eye’s choroidal thickness in this patient would be almost two standard deviations above the mean; while the left eye’s choroidal thickness would be three times above it. Second, the patient did not exhibit CSR risk factors such as corticosteroid use, Type A personality, or other medications associated with the disease.
Third, cataract surgery may exacerbate existing UES.1,4 Our patient self-reported a blurred spot post cataract extraction which would appear to support this correlation.
Finally, the peripapillary cystic spaces in both eyes were consistent with the fluid leakage theory in PUES proposed by others. All the above combined with the hyperopic status and thickened sclera (Figure 8) edged PUES to the more likely diagnosis.
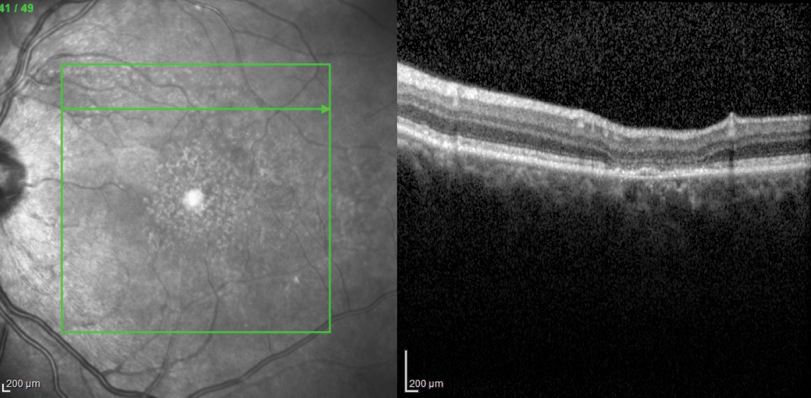 |
| Figs. 7. This OCT-B scan reveals the left eye’s outer retina and RPE atrophy. |
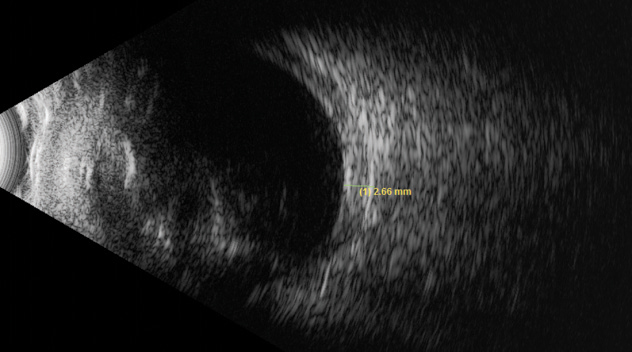 |
| Fig. 8. This ultrasound of the left eye shows a thickened retina-choroid-sclera complex, but no peripheral choroidal detachments. |
Treatment
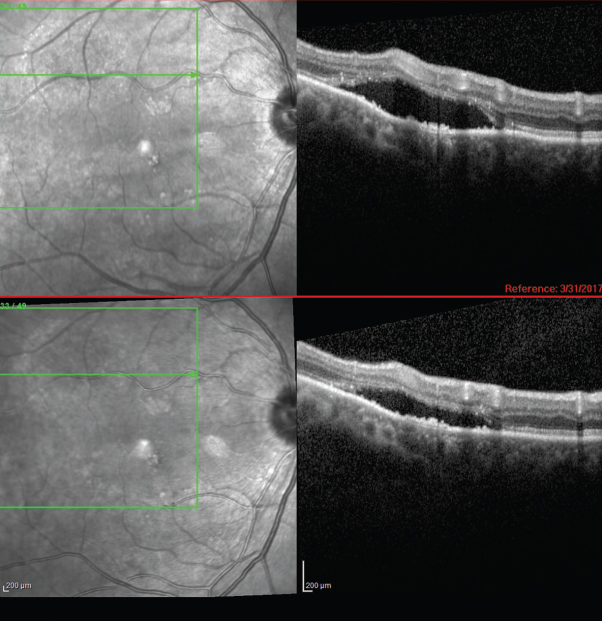
Both UES and PUES are difficult to manage and often follow a relapsing course.6 Full-thickness sclerectomy is the surgical treatment of choice by many for UES, but outcomes are variable.1 In their case of PUES, investigators reported effective treatment with oral acetazolamide for the acute stage, with a switch to the topical form for maintenance. They cited a 2009 literature review which shows posterior pole effects from topical carbonic anhydrase inhibitor therapy and theorized that since PUES is isolated to the posterior pole, this treatment may increase fluid transport from the subretinal space to the choroid.4 In this case, the patient declined any treatment and elected to observe the condition. At eight months, some areas of serous retinal detachment improved (Figure 4) while others remained relatively stable. The fovea remained flat with intact architecture OU.
UES and PUES can lead to serious and permanent vision loss, but prompt recognition of their characteristic clinical signs and appropriate ancillary testing will guide management strategies. Diagnosing UES or PUES necessitates the use of multimodal imaging and expanded critical thinking.
1. Haque R. Efficacy and safety of dexamethasone/povidone-iodine ophthalmic suspension in adenoviral conjunctivitis. Presented at: Association for Research and Vision in Ophthalmology annual meeting; May 7-11, 2017; Baltimore. |

