 |
Q:
I have a 55-year-old patient who has had chronic keratouveitis following a zoster outbreak for more than three and a half years. He currently uses low dose topical steroids and Valtrex 500mg (GlaxoSmithKline) daily and has inquired about the recently approved vaccine, Shingrix (GlaxoSmithKline), for shingles. Considering his chronic ocular disease from zoster, is he a candidate for the vaccine? If so, how do we proceed?
A:
In short, this patient is indeed a candidate for the October 2017 FDA-approved zoster vaccine, Shingrix—the newest zoster vaccine to be introduced to the market—says Stephanie Klemencic, OD, associate professor at the Illinois College of Optometry and the Illinois Eye Institute. Dr. Klemencic says patients are at a higher risk of developing recurrent zoster eye disease if they have not received a zoster vaccine. However, she recommends exercising caution, as episodes of herpes zoster (HZ) temporarily increase cell-mediated immunity to the varicella virus. She notes that current guidelines advise delaying vaccine use until the episode is resolved or, in the case of chronic disease, the disease is stable.1
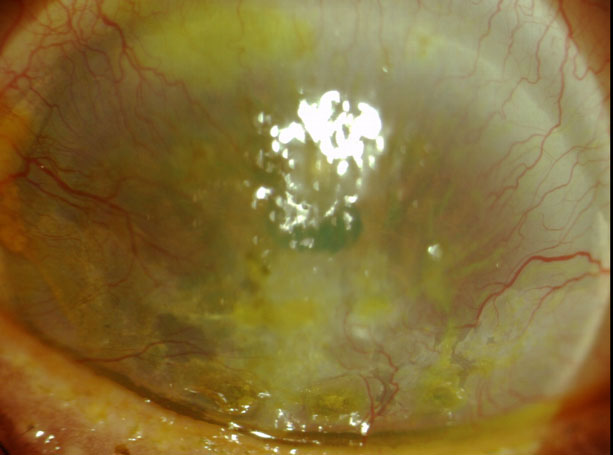 |
This patient has severe corneal neovascularization and interstitial keratitis due to chronic, recurrent HZO. |
Weighing Your Options
Within the weeks leading up to and following zoster vaccination, Dr. Klemencic suggests patients with a history of herpes zoster ophthalmicus (HZO) or who have chronic, stable HZO undergo a dilated eye exam to ensure there is no recurrent HZO. Luckily, the risk of reactivating eye diseases is more rare with Shingrix, which does not contain live varicella virus, compared with vaccines that have the live virus, such as Zostavax (Merck), according to Dr. Klemencic.
Since Shingrix does not contain live virus, Dr. Klemencic says another plus is that there is no need to discontinue antiviral usage because these medications have no effect on the non-live vaccine virus. If a patient receives Zostavax or another vaccine with live virus, however, she notes that they must discontinue anti-viral usage at least one day before and wait to resume usage for two weeks after vaccination.2
Shingrix is also more efficacious than Zostavax—Shingrix clinical trials found that the vaccine’s efficacy remained high at 84% over four years.3 The trials also discovered that the vaccine decreased the incidences of HZ and post-herpetic neuralgia by 97% and 91% for patients in their 50s and 60s, respectively.3 For patients 70 and older, it decreased the incidences of HZ and post-herpetic neuralgia by 91% and 88.8%, respectively.4
“No matter how you look at it, shingles is a bad disease,” says Dr. Klemencic. “As optometrists, we can play a pivotal role in preventing this disease (or decreasing the severity of its complications) by discussing the new zoster vaccination with our patients age 50 and older.” She adds that Shingrix is considered safe and should be administered to immunocompetent adults age 50 and older.
1. American Academy of Ophthalmology. Recommendations for herpes zoster vaccine for patients 50 years of age and older—2018. www.aao.org/clinical-statement/recommendations-herpes-zoster-vaccine-patients-50-. Published June 2018. Accessed November 15, 2018. 2. Immunization Action Coalition. Ask the Experts—Zoster (shingles). www.immunize.org/askexperts/experts_zos.asp. Published August 7, 2018. Accessed November 15, 2018. 3. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR. 2018;67(3):103-8. 4. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. NEJM. 2016;375:1019-32. |

