The use of contact lenses as ocular drug delivery systems was first introduced as an idea decades ago. As contact lenses have advanced, the once-futuristic thought of drug-delivery contact lenses is now becoming a reality. This article discusses how improvements in contact lenses have helped pave the way for a new wave of drug delivery systems.
Background
We are well aware that visual impairment and ocular disease are highly prevalent worldwide and can be debilitating. According to the National Eye Institute, the estimated number of people affected by the most common eye diseases will double between 2010 and 2050.1 These conditions include diabetic retinopathy, glaucoma, age-related macular degeneration and cataracts. Modern treatment modalities for ocular disease range from conventional liquid eye drops and ocular medications to invasive injections in the vitreous and surgical procedures to the removal of damaged areas and implant devices.2-4
 |
|
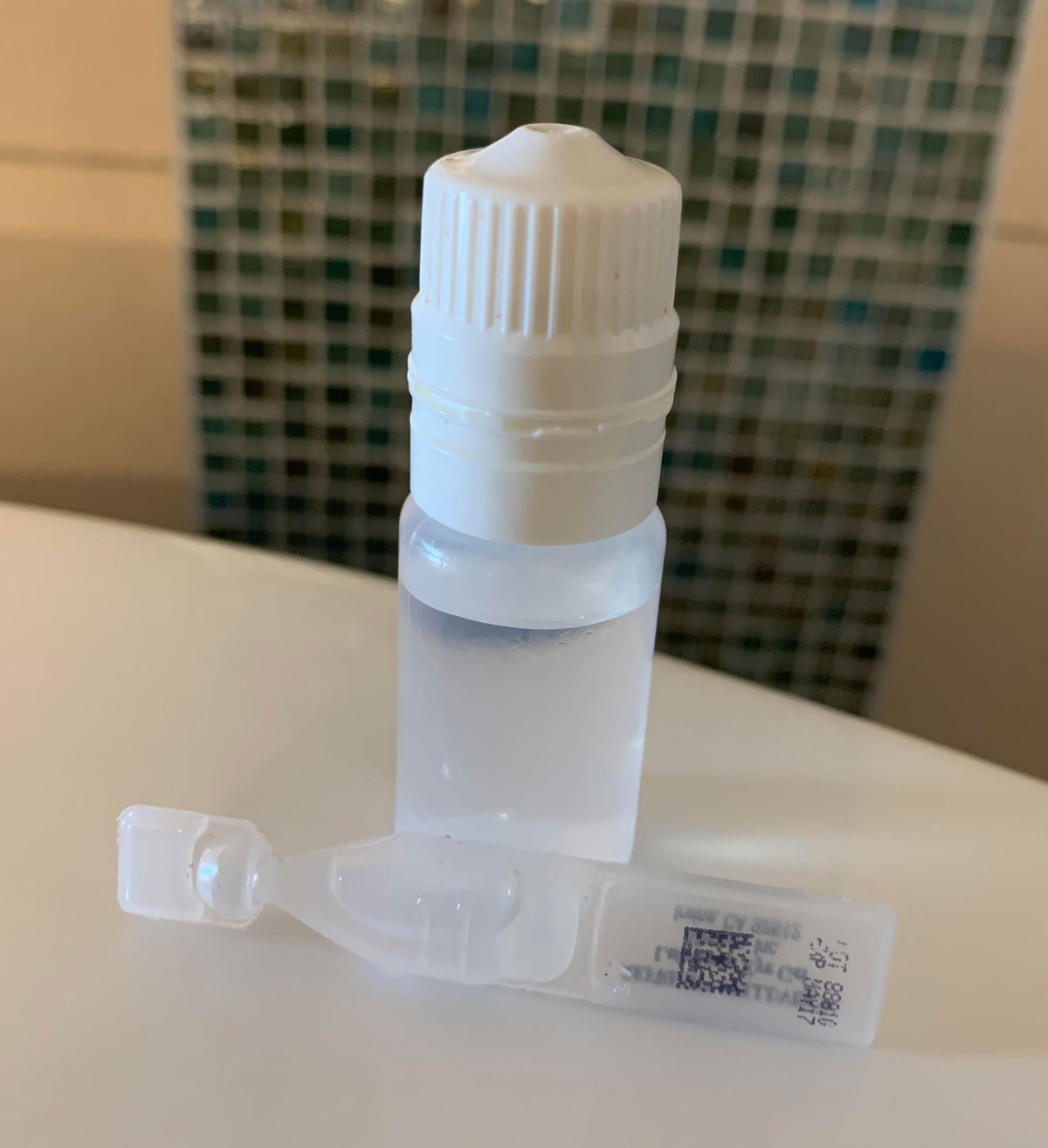
These mainstays of drug delivery can make drop instillation challenging for patients. Click image to enlarge. |
Eye drops have traditionally been the standard method for delivering medications to the eye. Unfortunately, a major disadvantage with eye drops is their low bioavailability of less than 5%.5 That’s not all. A landmark review illustrated that eye drops are associated with a pulsatile delivery, with a wide range of tissue concentrations.6 This variability is undesirable, particularly in the case of chronic treatment of glaucoma with molecules of short duration of action. Eye drops for long-term use come with the same risk associated with any chronic patient-administered medication, requiring treatment adherence.
With respect to the application of drops, patient adherence to therapy is impacted by a variety of issues, including drug cost, accessibility, availability, regimen, convenience, iatrogenic discomfort or irritation, dropper tip contamination and side effects.7 At times, poor communication or understanding of why the medication is recommended is the culprit of nonadherence.8 Equally as important is proper instillation of eye drops, which is only correctly performed by a smaller subset of patients.9-11 Systemic disease such as advanced rheumatoid arthritis, poor dexterity, tremor, reduced grip strength, loss or deformity of digits and poor aim may make eye drop instillation difficult and bring about product waste.
Eye drops tend to have excessive volume since one dose is usually 20µL to 50µL, larger than the precorneal space of approximately 7µL.12 Typically, approximately only 1% to 5% of applied drug is absorbed into the eye.13 Ocular drug delivery systems are designed to overcome the limitations of eye drops in various ways, including extended residence time, decreased pulsatile delivery, controlled delivery and enhanced local delivery to the posterior segment.
The application of scleral lenses as drug delivery devices has been illustrated with the advantage of a large fluid reservoir. Scleral lenses provide a protected environment in which the corneal surface is continuously bathed in preservative-free fluid. These lenses are inherently stable to provide lasting ocular penetration of a drug. The main detriments of scleral lenses include handling and cost.14
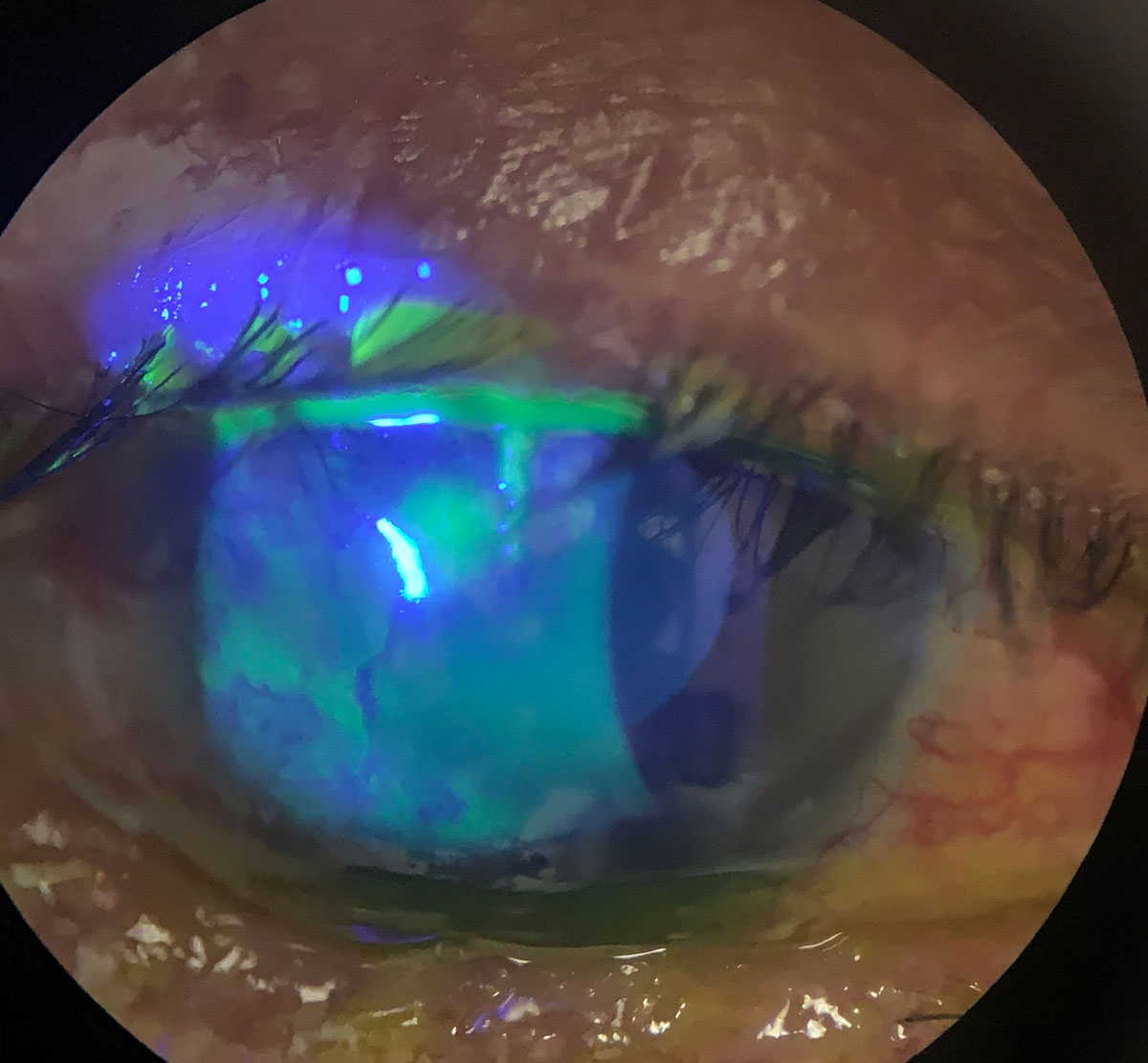
Various publications have reported the use of scleral lenses as ocular drug delivery systems. Specifically, corneal infiltrates have been treated with topical fortified preservative-free antibiotics in the bowl of the lens.15 Preservative-free antibiotics in the bowl of a continuously worn scleral lens have helped treat persistent epithelial defects.16 Anti-VEGF agents have been used in the bowl of a scleral lens to treat corneal neovascularization.17,18 In addition, stem cells on a scleral lens carrier have been used in the management of chemical burns in an animal model.19
 |
|
Antibiotics in the bowl of a scleral could help heal persistent epithelial defects and neurotrophic keratitis, as seen in this eye. Click image to enlarge. |
Design Methodology
There are a number of different methodologies that can be used to develop therapeutic contact lenses, each with their own advantages and disadvantages.
Soaking method. This simple, cost-effective approach involves soaking the contact lenses in a drug solution, which is then followed by drug uptake and release in the pre- and post-lens tear film.20 There are several factors that impact the drug reservoir’s ability, including water content, lens thickness, molecular weight of the drug, soaking time and the concentration of drug in the soaking solution.
Significant research has been conducted in this area. One study, which explored the uptake and release of timolol maleate and brimonidine tartrate using the soaking method, found that this drug delivery system may be a feasible method to control intraocular pressure (IOP) among glaucoma patients.21 The researchers found that 30 minutes of wear time per day for two weeks led to a reduction in IOP. This treatment corresponds to a 10x lower dose of eye drops.
There are limitations to this approach that must also be considered. Research has shown that drugs or polymers of a high molecular weight—such as hyaluronic acid—do not penetrate the aqueous channels of contact lenses. As a result, this approach has not proven effective for the treatment of dry eye.20 Another challenge is the low affinity that contact lenses have demonstrated for the majority of ophthalmic drugs such as timolol maleate, olopatadine hydrochloric acid and brimonidine tartrate. Lenses retain these drugs poorly and release them quickly, followed by a sharp decline.
Molecular imprinting. This technique, which uses hydrogel contact lenses, combines the drug with functional monomers that rearrange and interact with drug molecules. Following polymerization, the drug is removed from the contact lens, resulting in macromolecular memory sites with high drug affinity and increased drug loading capacity.20 The drug release pattern can be tailored based on the monomer composition. While this approach has promise, there are limits to its use. The highly crosslinked structure of the hydrogel impacts both the optical and physical performance of the contact lens. Extended wear is also limited due to insufficient ion and oxygen permeability caused by a decrease in water content.20
Colloidal nanoparticle-laden lenses. This method creates nanoparticle-loaded contact lenses that can deliver drugs at a controlled rate over an extended period of time. Using various colloidal nanoparticles, researchers have developed therapeutic contact lenses that not only offer extended drug delivery but also comfortability.20 For example, various efforts have been focused on polymeric nanoparticles. One research team looking at treatment for glaucoma incorporated timolol-loaded propoxylated glyceryl triacylate nanoparticles in contact lenses. The in vitro release profile showed that the drug was present for one month.22 Additionally, animal studies demonstrated a reduction in IOP.23 However, a reduction in ion and oxygen permeability as well as an increase in storage modulus was also observed.
Cyclodextrins have been used to achieve continued delivery of hydrophobic agents. Data from an animal study demonstrated an increase in drug residence time. The researchers also observed a higher concentration of the drug in the tear fluid and vitreous humor when compared with conventional hydrogel lenses and eye drops.24
Liposomes, which are biocompatible and biodegradable, have a variety of drug delivery applications, and extensive research has been done to better understand their potential for therapeutic contact lenses. In one study, researchers encapsulated lidocaine-loaded dimyristoyl phosphatidylcholine liposomes in a contact lens and found that lidocaine is released for approximately eight days.20 Other data found that hydrogel lenses with two layers of liposomes released the drug for up to 30 hours. Comparatively, 10 layers demonstrated drug release for as many as 120 hours.25 It is important to note that, while these results have potential, multilayer liposomes decreased the oxygen and carbon dioxide permeability of the contact lenses.
Another promising avenue of study involves microemulsion and micelles, which have potential due to their thermodynamic stability, high drug-loading capacity, increased wettability and ability to easily tailor the drug release pattern.20
 |
|
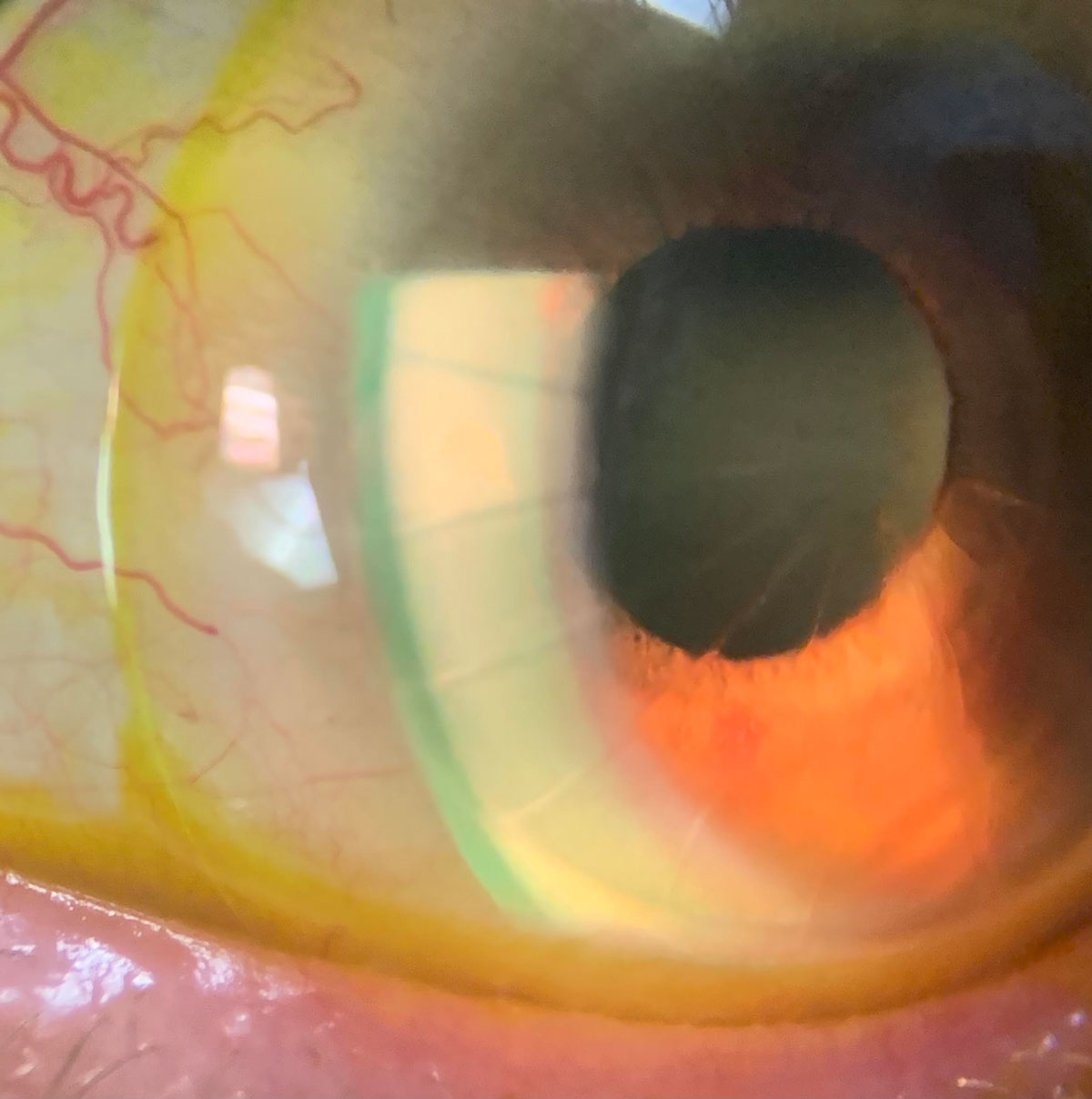
A scleral with sodium fluorescein on an eye with radial keratectomy, lipid keratitis and neovascularization. Click image to enlarge. |
Use of vitamin E. To address some of the limitations associated with drug-eluting contact lenses, the role of vitamin E is under investigation. In addition to being biocompatible, vitamin E is hydrophobic and exhibits low water solubility. And so, it has been used to slow down the rate at which a drug is released.26
One study showed that the release of timolol was significantly extended by increasing vitamin E loading; however, it also posed a challenge to oxygen and ion permeability.20 Another team of researchers created dexamethasone contact lenses with 30% vitamin E loading, which extended the drug-release duration for nine days.27
While the addition of vitamin E holds promise as a means to slow the release of several hydrophilic agents, it has its own limitations that must be taken into consideration. These include a reduction in ion and oxygen permeability and an increase in storage module and protein adsorption due to its hydrophobic properties.20
New and Future Developments
With ongoing research and a growing understanding of the potential clinical applications, advancements in contact lens drug delivery systems continue. Recently, the first lens for drug delivery was approved in Japan and Canada. Called Acuvue Theravision with Ketotifen (Johnson & Johnson Vision), it is a daily disposable lens for patients who experience itchy eyes due to allergic conjunctivitis.28 FDA trials of the lens are ongoing.
Up to 20% of the US population experiences ocular allergies. Globally, the prevalence is similar. Results from two Phase III trials evaluated the antihistamine-releasing contact lens (etafilcon A with 0.019mg ketotifen) demonstrated that patients who wore the lenses had lower mean itching scores following exposure to allergens compared with those wearing non-medicated lenses.29 In clinical trials, itching was prevented for up to 12 hours.30 The approval of this novel contact lens delivery system is a significant advancement, highlighting the potential to simultaneously correct vision and provide therapeutic interventions for contact lens wearers.
 |
|
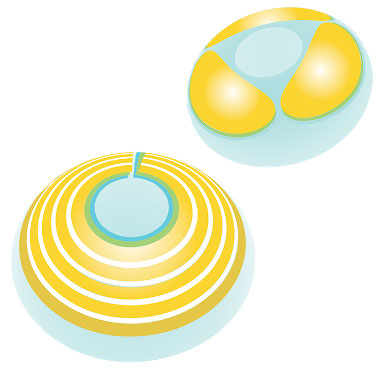
The MediPrint printing process can print multiple layers surrounding the optical zone. The layers may contain the same or different drugs; each layer may have diverse release kinetics. Click image to enlarge. |
Another promising development in contact lens drug delivery is the SIGHT (Sustained Innovative Glaucoma and Ocular Hypertension Treatment) clinical program, which seeks to treat mild to moderate glaucoma and ocular hypertension. The Phase IIa SIGHT-1 trial evaluated LLT-BMT1 (MediPrint Ophthalmics), a drug-eluting lens for glaucoma treatment that uses the FDA-approved drug bimatoprost.31 This process allows for the printing of drug and barrier layers on the lens surface to control the diffusion release kinetics of drugs. Five patients underwent treatment wearing an LLT-BMT1 lens in each eye for seven days continuously. Study participants were neophyte contact lens wearers with an average age of 77.4.
The study demonstrated strong safety signals with 100% tolerability and no significant adverse events. The researchers also found that the incidence of hyperemia among study participants was lower than what is observed for bimatoprost drops—a standard of care approach for this condition.31 The SIGHT-1 data also indicated that a single dose has efficacy, which led to the initiation of SIGHT-2, a larger Phase IIb study. There are also plans for a Phase III study to further explore the potential of this treatment approach.
Researchers are also exploring latanoprost-eluting contact lenses as a means to lower IOP in glaucoma patients. Preclinical data has shown that continued delivery of latanoprost via contact lenses is at least as effective as daily latanoprost ophthalmic solution.32 This efficacy study of glaucomatous monkeys evaluated latanoprost-eluting low- and high-dose contact lenses. The researchers reported that latanoprost ophthalmic solution led to an IOP reduction of 5.4±1.0mm Hg on day three and a peak IOP reduction of 6.6±1.3mm Hg on day five. Comparatively, latanoprost-eluting low-dose contact lenses lowered IOP by 6.3±1.0mm Hg, 6.7±0.3mm Hg and 6.7±0.3mm Hg on days three, five and eight, respectively. The high-dose lenses reduced IOP by 10.5±1.4mm Hg (day three), 11.1±4.0mm Hg (day five) and 10.0±2.5mm Hg (day eight).32 Further study is necessary to better understand the safety, efficacy and ideal dosage of this system.
Patients who undergo ocular surgery, such as cataract or LASIK, must follow strict postoperative guidelines to avoid complications. This often includes using eye drops; however, as previously discussed, they are not always administered properly or at the required frequency. Contact lenses that deliver anti-inflammatory, antibiotic and pain-reducing drugs evenly over time are currently under investigation as a way to improve postoperative care for these patients as well as those who suffer from corneal abrasions.33
Another area where drug-eluting contact lenses could have significant impact is in the treatment of ocular inflammation—a leading cause of blindness. Currently, the standard of care is topical ophthalmic solutions, such as dexamethasone eye drops. However, the side effects as well as a need for frequent administration can make adherence challenging.
In an effort to find a better delivery system, researchers developed a dexamethasone-releasing contact lens. Animal models have demonstrated this approach is a safe and effective treatment for anterior ocular inflammation.34 The researchers reported that the lenses inhibited suture-induced corneal neovascularization and inflammation for seven days and lipopolysaccharide-induced anterior uveitis for five days. While more research is needed, dexamethasone-eluting lenses could prove to be an effective treatment for ocular inflammation and a promising drug delivery system.
Clinical Applications
As far as in-clinic application goes, there are multiple management strategies for patients with dry eye disease, seasonal ocular allergies, glaucoma or ocular infection, for example. In general, it is usually safe to initiate the following protocol and proceed according to your findings on a case-by-case basis. Prior to the ocular examination, provide a questionnaire about ocular symptoms to better inform the evaluation process. Upon identification of the patient’s condition, offer various management options, including drug-delivery contact lenses, and review the risks and benefits of each.
If both the practitioner and patient opt to go the drug-delivery contact lens route and the patient is not an existing wearer, conduct lens application and removal training, review the lens wearing schedule and provide written instructions to the patient. Soft drug-delivery contact lenses can usually be dispensed the same day. However, a scleral drug-delivery contact lens may take a few days to manufacture.
After initiating this form of therapy, schedule a follow-up visit to review the therapeutic effects of the drug-delivery contact lens, contact lens handling and patient compliance.
Takeaways
Drug-eluting contact lenses are poised to become a viable alternative therapeutic option for various ocular diseases and beyond. As advancements continue, doctors and their patients will be able to experience firsthand the impact these systems can have on overall health and quality of life.
Dr. Barnett is a principal optometrist at the University of California, Davis Eye Center in Sacramento and Davis, CA. She is chair of the American Optometric Association Contact Lens and Cornea Section, a fellow of the American Academy of Optometry, a diplomate of the American Board of Certification in Medical Optometry, a fellow and global ambassador of the British Contact Lens Association and a board member of both the Gas Permeable Lens Institute and the International Society of Contact Lens Specialists. Dr. Barnett is a consultant for Johnson & Johnson.
1. National Eye Institute. Eye health data and statistics. July 17, 2019. nei.nih.gov/eyedata. Accessed July 8, 2021. 2. Abell RG, Darian-Smith E, Kan JB, et al. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: outcomes and safety in more than 4000 cases at a single center. J Cataract Refract Surg. 2015;41(1):47-52. 3. Chuang AT, Margo CE, Greenberg PB. Retinal implants: a systematic review. Br. J Ophthalmol. 2014;98(7):852-6. 4. Zhang YX, Chen YF, Shen XY, et al. Reduction- and pH-sensitive lipoic acid-modified poly(L-lysine) and polypeptide/silica hybrid hydrogels/nanogels. Polymer. 2016;86:32-41. 5. Jones L, Hui A, Phan CM, et al. CLEAR - Contact lens technologies of the future. Cont Lens Anterior Eye. 2021;44(2):398-430. 6. Shell JW. Ophthalmic drug delivery systems. Surv Ophthalmol. 1984;29(2):117-28. 7. Hennessy AL, Katz J, Covert D, et al. Videotaped evaluation of eyedrop instillation in glaucoma patients with visual impairment or moderate to severe visual field loss. Ophthalmology. 2010;117(12):2345-52. 8. Slota C, Sayner R, Vitko M, et al. Glaucoma patient expression of medication problems and nonadherence. Optom Vis Sci. 2015;92(5):537-43. 9. Stone JL, Robin AL, Novack GD, et al. An objective evaluation of eye-drop instillation in glaucoma patients. Arch Ophthalmol. 2009;127(6):732-6. 10. Kass MA, Hodapp E, Gordon M, et al. Patient administration of eyedrops: observation. Part II. Ann Ophthalmol. 1982;14(9):889-93. 11. Kass MA, Hodapp E, Gordon M, et al. Part I. Patient administration of eyedrops: interview. Ann Ophthalmol. 1982;14(8):775-9. 12. Mishima S, Gasset A, Klyce SD, et al. Determination of tear volume and tear flow. Invest Ophthalmol Vis Sci. 1966;5:264-76. 13. Tang-Liu DD, Liu S, Neff J, et al. Disposition of levobunolol after an ophthalmic dose to rabbits. J Pharm Sci. 1987;76(10):780-3. 14. Johns LK, McMahon JA, Barnett M. Contemporary Scleral Lenses: Theory and Application. Vol 4. Bentham Science; 2017:201-41. 15. Kalwerisky K, Davies B, Mihora L, et al. Use of the Boston Ocular Surface Prosthesis in the management of severe periorbital thermal injuries: a case series of 10 patients. Ophthalmology. 2012;119(3):516-21. 16. Ciralsky JB, Chapman KO, Rosenblatt MI, et al. Treatment of refractory persistent corneal epithelial defects: a standardized approach using continuous wear PROSE therapy. Ocul Immunol Inflamm. 2015;23(3):219-24. 17. Keating AM, Jacobs DS. Anti-VEGF treatment of corneal neovascularization. Ocul Surf. 2011;9(4):227-37. 18. Lim M, Jacobs DS, Rosenthal P, et al. The Boston Ocular Surface Prosthesis as a novel drug delivery system for bevacizumab. Semin Ophthalmol. 2009;24(3):149-55. 19. Espandar L, Caldwell D, Watson R, et al. Application of adipose-derived stem cells on scleral contact lens carrier in an animal model of severe acute alkaline burn. Eye Contact Lens. 2014;40(4):243-7. 20. Maulvi FA, Soni TG, Shah DO. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016;23(8):3017-26. 21. Schultz CL, Poling TR, Mint JO. A medical device/drug delivery system for treatment of glaucoma. Clin Exp Optom. 2009;92(4):343-8. 22. Jung HJ, Chauhan A. Extended release of timolol from nanoparticle-loaded fornix insert for glaucoma therapy. J Ocul Pharmacol Ther. 2013;29(2):229-35. 23. Jung HJ, Abou-Jaoude M, Carbia BE, et al. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J Control Release. 2013;165(1):82-9. 24. Xu J, Li X, Sun F. Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater. 2010;6(2):486-93. 25. Danion A, Arsenault I, Vermette P. Antibacterial activity of contact lenses bearing surface-immobilized layers of intact liposomes loaded with levofloxacin. J Pharm Sci. 2007;96(9):2350-63. 26. Holgado MA, Anguiano-Domínguez A, Martín-Banderas L. Contact lenses as drug-delivery systems: a promising therapeutic tool. Arch Soc Esp Oftalmol (Engl Ed). 2020;95(1):24-33. 27. Kim J, Peng CC, Chauhan A. Extended release of dexamethasone from silicone-hydrogel contact lenses containing vitamin E. J Control Release. 2010;148(1):110-6. 28. Cision PR Newswire. Johnson & Johnson Vision receives approval in Canada for world’s first and only drug-releasing contact lens for vision correction and allergic eye itch: Acuvue Theravision with Ketotifen. April 27, 2021. www.prnewswire.com/news-releases/johnson--johnson-vision-receives-approval-in-canada-for-worlds-first-and-only-drug-releasing-contact-lens-for-vision-correction-and-allergic-eye-itch-acuvue-theravision-with-ketotifen-301277041.html. Accessed July 8, 2021. 29. Pall B, Gomes P, Yi F, et al. Management of ocular allergy itch with an antihistamine-releasing contact lens. Cornea. 2019;38(6):713-7. 30. Johnson & Johnson Vision. ACUVUE Theravision with Ketotifen. www.jnjvisionpro.ca/products/acuvue-theravision. Accessed July 8, 2021. 31. Busines Wire. MediPrint Ophthalmics announces successful completion of its SIGHT-1 Phase 2a clinical study. March 1, 2016. www.businesswire.com/news/home/20210316005609/en/MediPrint™-Ophthalmics-Announces-Successful-Completion-of-Its-SIGHT-1-Phase-2a-Clinical-Study. Accessed July 8, 2021. 32. Ciolino JB, Ross AE, Tulsan R, et al. Latanoprost-eluting contact lenses in glaucomatous monkeys. Ophthalmology. 2016;123(10):2085-92. 33. OCU Medic. New drug-eluting contact lens to provide timed drug delivery directly to eye; improving recovery, reducing cost for millions after eye surgery and LASIK. May 15, 2018. ocumedic.us/new-drug-eluting-contact-lens-to-provide-timed-drug-delivery-directly-to-eye-improving-recovery-reducing-cost-for-millions-after-eye-surgery-and-lasik/. Accessed July 8, 2021. 34. Bengani LC, Kobashi H, Ross AE, et al. Steroid-eluting contact lenses for corneal and intraocular inflammation. Acta Biomater. 2020;116:149-61. |

