Eye care is a vast field, and optometrists can’t be expected to be experts in everything. Many ODs choose to specialize in a certain type of care, such as pediatrics or anterior segment, while shying way from others, such as scleral lens fitting and myopia control. The same can be true in managing ocular disease states such as glaucoma and retina.
But dry eye shouldn’t be one of them. According to The Tear Film and Ocular Surface Society’s Dry Eye Workshop II (TFOS DEWS II), signs of dry eye can occur in up to 75% of some populations, and its prevalence only increases with age.1 Severe dry eye—disease that has a major impact on quality of life—can affect as much as 10% of the population older than age 50.1 Faced with such numbers and patient impact, we should all be prepared to care for dry eye patients at all stages of the disease, especially when it becomes detrimental to our patient’s quality of life. A solid understanding of dry eye is the foundation of care, but knowing how to navigate the complex treatment options is just as important. This article will help you better understand the many dry eye treatment regimens that go beyond the basics.
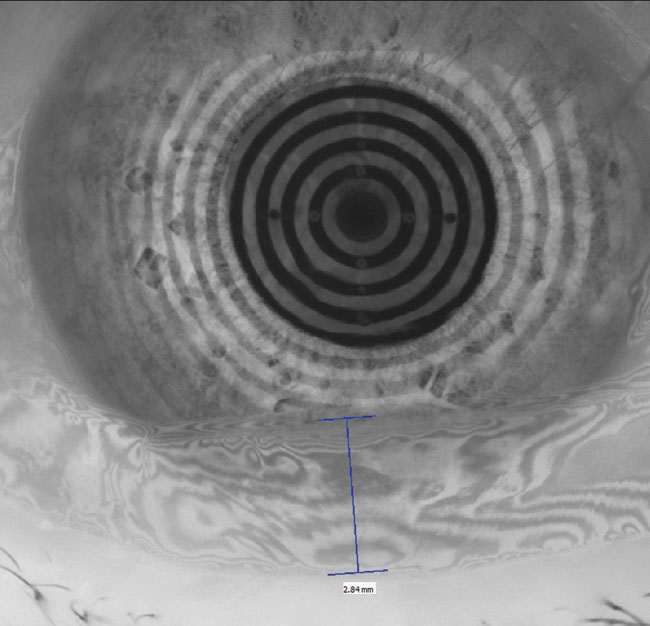
 |
| Fig. 1. The restoration of a smooth corneal and refractive surface can be seen in these before, at left, and after placido ring reflections after amniotic membrane therapy. Click image to enlarge. |
Where to Start
Severe dry eye is a large umbrella term for ocular surface conditions that are difficult to manage and are unresponsive to traditional first-line therapies such as artificial tears and at-home warm compresses. TFOS DEWS II came to a consensus on classifying dry eye subtypes and a diagnostic test battery—both of which can help you better understand this complex disease and how to proceed with advanced therapies when necessary.2 As an example, patients with a mixture of evaporative and aqueous deficient signs are often simultaneously flagged as abnormal or borderline during dry eye testing. These patients may also have Demodex blepharitis and allergic conjunctivitis, worsening the symptoms and inflammatory environment on the ocular surface.
For all patients presenting with suspicious signs or symptoms, a logical place to start is a validated symptom survey or questionnaire. DEWS II emphasizes the utility of the Ocular Surface Disease Index (OSDI) and the 5-Item Dry Eye Questionnaire 5 (DEQ-5). A highly cited study suggests an OSDI score of 33 to 100 indicates severe disease, while a minimal clinically important difference, whether better or worse, is 7.3 to 13.4 for severe disease.1 When using the DEQ-5, researchers suggest a cutoff point of ≥6 for keratoconjunctivitis sicca and ≥12 as suspicious for Sjögren’s syndrome (SS).3 Once the patients are flagged for severe dry eye using either questionnaire, the management can then be tailored based on the severity, response or non-response to past interventions, comorbidities and the patient’s visual goals.
Healing, One Step at a Time
Many options now exist to restore homeostasis to the tear film composition in severe dry eye—and initiate ocular surface healing. For many patients, clinicians should consider a step-wise approach to therapy, beginning with immediate relief with topical artificial tears, and escalating as the case deems necessary.
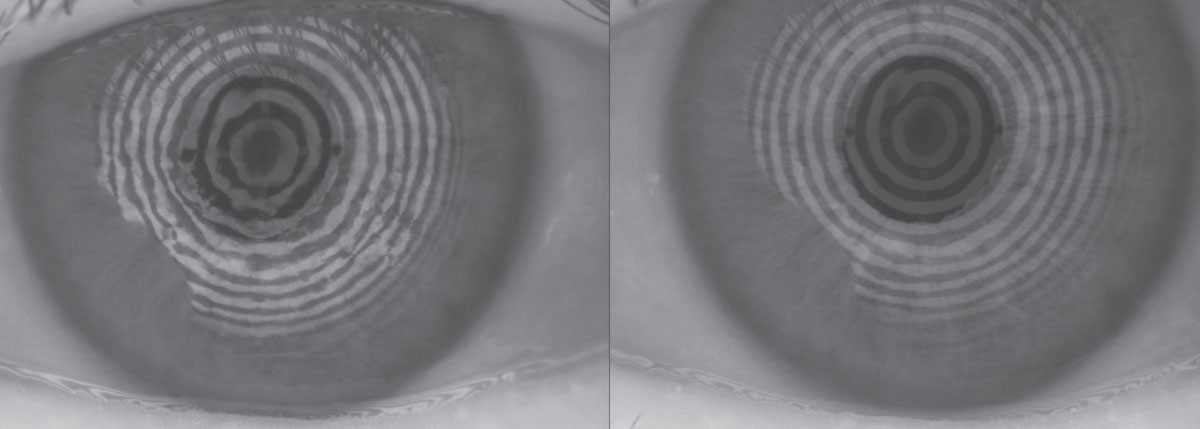
 |
| Fig. 2. This patient with 75% meibomian gland atrophy may benefit from in-office MG expression. Click image to enlarge. |
Artificial tears. These over-the-counter drops remain the first-line treatment, even for severe dry eye. Myriad options offer a wide array of active and inactive ingredients—all of which with the ultimate goal of supplementing the natural tear film and diluting it to reduce the osmolarity, known to cause ocular surface damage. Some newer options on the market may provide more tailored relief for patients with severe symptoms.
The recently released Systane Complete (Alcon) has twice the concentration of propylene glycol (0.6% vs. 0.3%) compared with the company’s Systane Ultra and contains the micro-emulsions of mineral oil from its Systane Balance. This product uses nano-droplet technology designed for longer retention time and thus prolonged and uniform protection of the ocular surface.4
Trehalose—a sugar present in some plants that gives them the ability to survive in harsh environments without water—is incorporated as an osmoprotectant into both Refresh Optive Mega-3 (Allergan) and Thera Tears Extra (Akorn). In a study of various artificial tears with stressed human corneal epithelial cells, trehalose-based eye drops showed the highest efficiency in prevention of cell death from dessication.5
Retaine MGD (OcuSoft) owes much of its success to its cationic nanoemulsion properties, taking advantage of the inherent negative charge of the ocular surface for spreading and retention.6
Clinicians must keep in mind that dosing any artificial tear too frequently can strip the ocular surface of its naturally occurring beneficial components (i.e., lactoferrin and lysozyme) and worsen the condition.7 The use of artificial tears should be thought of as a preventative measure prior to the onset of symptoms and prior to activities that challenge the fragile tear film.
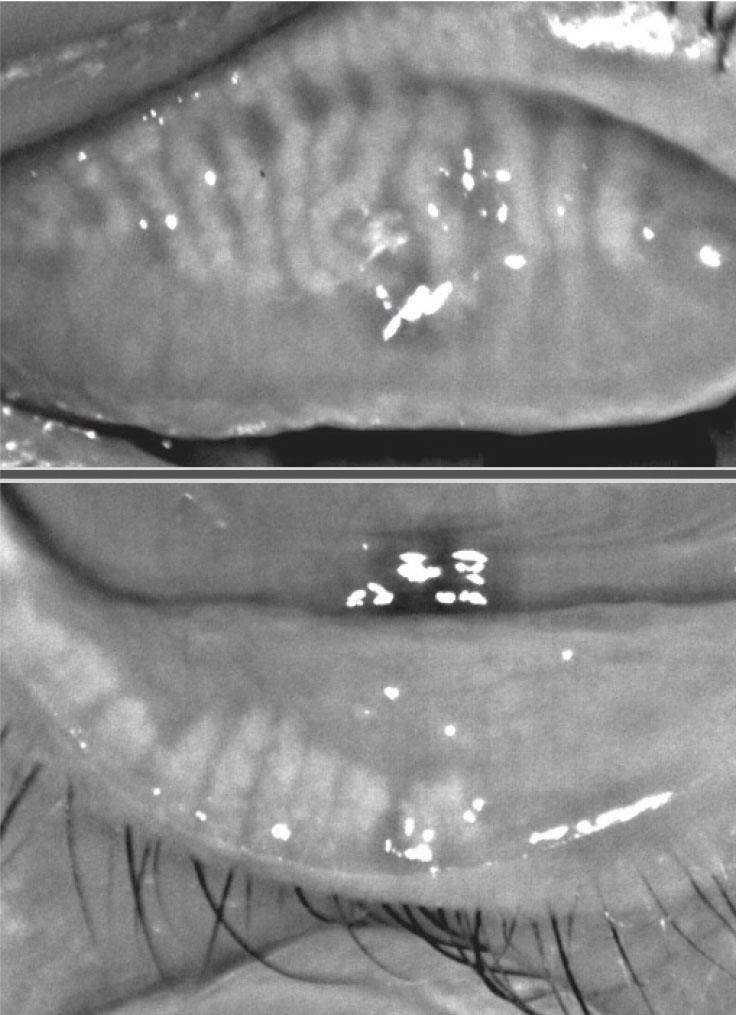
 |
| Fig. 3. This patient, seen here before (at left) and after treatment, experienced significant improvement in signs and symptoms of Demodex infestation with four months of tea tree oil foam cleanser QHS. Click image to enlarge. |
Punctal plugs. For severe dry eye patients whose symptoms are exacerbated by excessive tear drainage, punctal plugs may help. Lacrimal occlusion, whether temporary, semi-permanent or permanent, can increase tear volume and contact time of natural tears on the ocular surface. Studies show this treatment method can improve patient-reported symptoms of dry eye, and the procedure is considered both safe and effective compared with artificial tear use alone. However, clinicians should be careful to only use this intervention for patients with drainage system dysfunction; if that is not a contributing factor, occlusion may do more harm than good.8-10
Autologous serum. Derived from the patient’s own blood, this lab-created drop provides ingredients and properties, such as pH and osmolarity, biochemically similar to that of healthy tears.11 The serum contains several epithelial and nerve growth factors, including vitamin A, epidermal growth factor and fibronectin—all of which can be helpful in nerve regeneration.12 To offer this management strategy, eye care providers need to have a local laboratory that partners with a compounding pharmacy. Patients have approximately 30 vials of blood drawn, tested for disease and then diluted to the appropriate concentration. Due to its sterile and unpreserved nature, bottles yet to be used must be stored frozen and thawed one at a time. While one review was inconclusive, a retrospective cohort study of 63 patients using autologous serum for three to 48 months found improvements in corneal fluorescein staining, Schirmer scores and symptoms.13,14
New Drops in the PipelineDespite their clinical utility, topical drops come with considerable barriers. Instillation is dependent on the dexterity and skillfulness of the patient, and drops usually wash away within 30 seconds, allowing less than 5% to reach the intraocular tissues.22 Luckily, advances are helping improve this treatment modality. Nanomicelles are excellent pharmaceutical carriers because of their ability to minimize drug degradation, lower adverse side effects and improve drug permeation through the ocular epithelium with minimal or no irritation, ultimately leading to enhanced ocular bioavailability.21 Much of this activity stems from the nanomicelles having both hydrophilic and hydrophobic properties. A Phase III confirmatory study of a preservative-free aqueous formulation of cyclosporine A 0.09% that uses nanomicelles was performed in 744 dry eye patients with promising results. After 12 weeks of therapy, the drug provided statistically significant improvement in Schirmer’s score compared with vehicle alone.28 Another novel eye drop molecule is perfluorohexyloctane, which research shows has a cumulative effect of thickening the lipid layer 13.36% over four weeks compared with a minimal 3.21% of saline. Current investigations suggest its benefits derive from semifluorinated alkanes’ ability to rapidly spread on the ocular surface, creating a significantly larger volume-adjusted spread area than saline with a contact angle of almost 0°.29 |
Amniotic membranes. When chronic insult to the ocular surface results in persistent corneal epithelial defects, amniotic membranes can protect and quickly restore these areas. Research shows complete or partial success in the vast majority of various ocular surface disorders.15 It is important to dose a prophylactic topical antibiotic to minimize the risk of a secondary bacterial infection because these patients are spending two or more nights in a closed-eye state (Figure 1).
Amniotic eye drops. These are another advanced therapy that contains the beneficial properties of autologous serum and amniotic membranes. While dosing and efficacy have not been well studied, amniotic eye drops are thought to deliver their healing effects in a more patient-accessible medium compared with an amniotic membrane.
Scleral lenses. As an efficacious treatment strategy for severe dry eye, these lenses constantly bathe the ocular surface with sterile saline and protect it from the external environment.16 They should be employed when the patient’s tear film volume is low or the quality is poor to the point of instant evaporation and exposure. Clinicians should select a larger diameter (i.e., ≥16.0mm) to cover more of the exposed cornea and conjunctiva. Because doing so will likely encounter more scleral toricity, toric scleral landing curves or further customization can help to promote good centration and minimize post-lens fogging.
Intranasal tear neurostimulation. This new therapy option, recently introduced as TrueTear (Allergan), works on the principle that neurostimulation will help the patient make more tears. A 180-day study in 40 subjects revealed that the device improved corneal staining by roughly one grade level and conjunctival staining by about two grade levels on the modified Oxford Scale.17 While tear break-up time did not change, Schirmer increased from 13mm to 20mm compared with unstimulated patients at day 180. Symptoms by OSDI decreased 53%, and each stimulation had an average duration of symptom relief of three hours with a time interval of six hours between each stimulation in a given day.
Another study found neurostimulation can lead to degranulation of goblet cells in the conjunctiva, which releases the natural complex substance and may directly improve the mucin layer.18
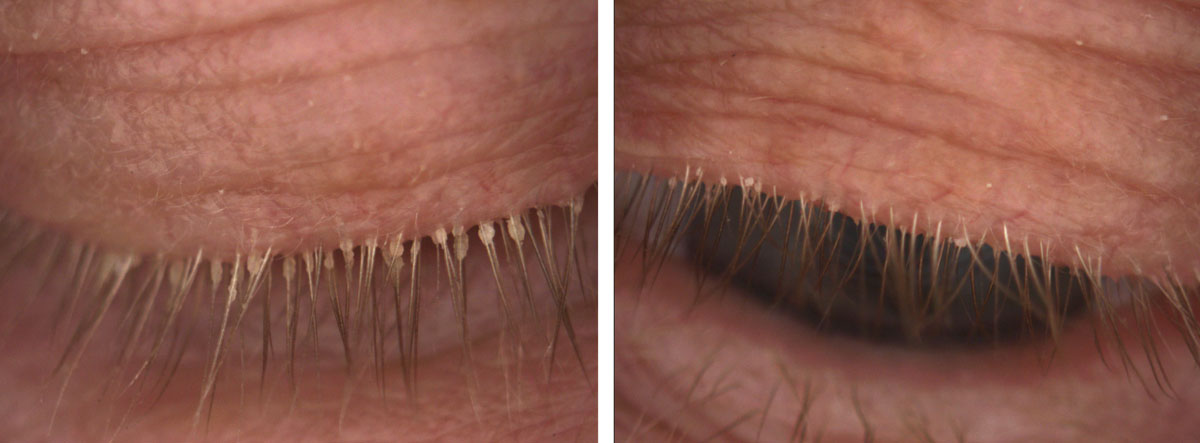
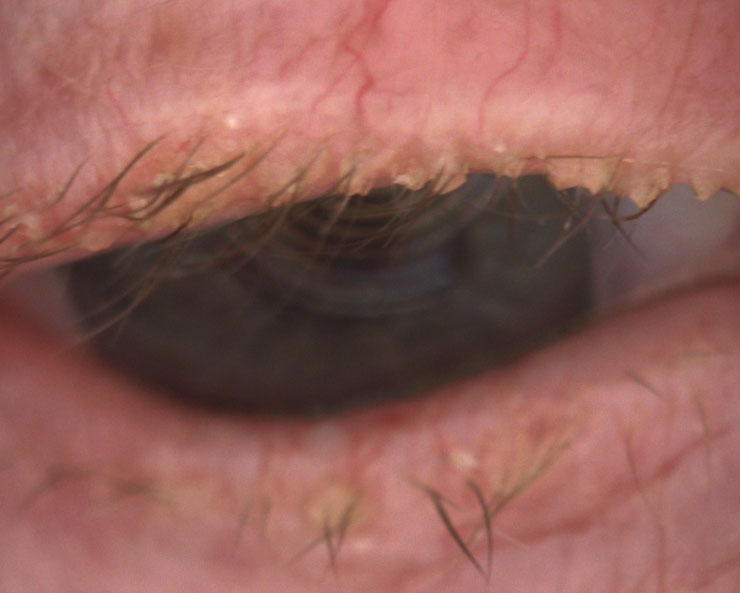 |
| Fig. 4. This 85-year-old female has end-stage Demodex, cylindrical dandruff and madarosis. |
In-office meibomian gland (MG) expression. Either manually with a Mastrota paddle or with a device such as Lipiflow (TearScience) or the recently approved iLux (Tear Film Innovations), this is an advanced option clinicians can employ to help treat severe dry eye from meibomian gland dysfunction (MGD). Clearing blocked MGs can help promote normal blink patterns and facilitate spread of a lower viscosity and healthier meibum (Figure 2).
When MGD stems from an inflammatory dermatological condition such as ocular rosacea, intense pulsed light (IPL) may help to reduce signs and symptoms. IPL’s accidental discovery for MGD was due to patients reporting fewer dry eye symptoms after upper cheek treatment for acne, rosacea and abnormal blood vessels.19 One study found tear break-up time improvement and patient-reported satisfaction after a series of IPL treatments.20
Specialty Cases
Often, severe dry eye can be linked to a comorbidity that exacerbates symptoms. Clinicians should include these in their differentials when honing the dry eye diagnosis, and tailor therapy accordingly when they arise:
Demodex continues to be underdiagnosed, despite its prevalence of 17.7% in young adults and up to 100% in older adults.17,19 From the initial diagnosis, photographing the entire upper eyelash area will not only drive patient compliance but also help monitor the treatment results (Figure 3).
Reducing Demodex counts can be accomplished with a variety of methods, the basis of which is tea tree oil. Eye care providers can prescribe a daily tea tree oil foam cleanser or single use packet lid wipe.
A recent study found similar efficacy between three treatments; the 86 subjects were randomly assigned tea tree face wash, OcuSoft Lid Scrub Plus and in-office debridement with the BlephEx lid cleaning device, and symptoms and number of Demodex folliculorum from one epilated lash all improved similarly.21
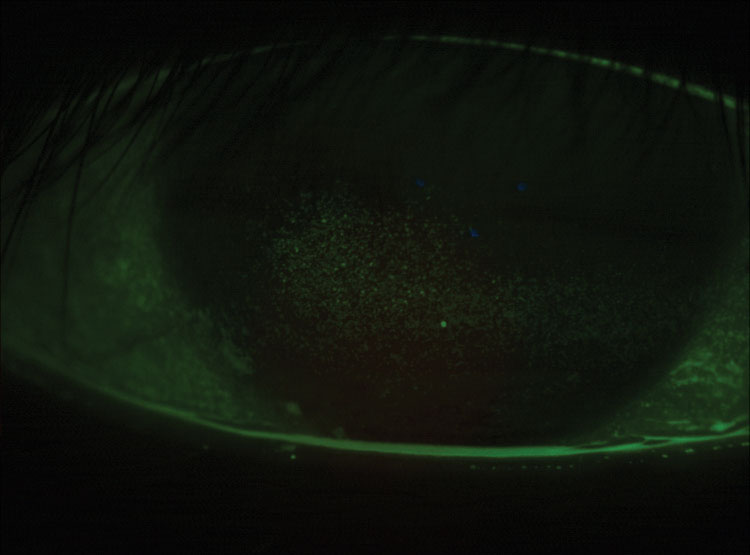 |
| Fig. 5. This a post-LASIK cornea has grade 3.5 coalesced superficial punctate keratitis. |
Severe cases may manifest with pervasive cylindrical dandruff that can be thick and sticky, potentially blocking the penetration of tea tree oil into the eyelash follicle (Figure 4). These cases may require a softening of the cylindrical dandruff with a foam cleanser or oil followed by manual removal of the stubborn and large accumulation of keratinization and lipids with forceps. In-office use of commercially available 50% tea tree oil may be necessary.22 Clinicians should use a topical anesthetic on the ocular surface prior to treating or even apply a silicone hydrogel contact lens prior to treatment to protect the cornea and soak up any residual tea tree oil on the front surface. Patients with punctal plugs may require an extra rinse to minimize the contact time between residual tea tree oil and the ocular surface epithelium.
Systemic diseases can also complicate a dry eye diagnosis and warrant significant follow up and continued communication with other care providers such as primary care doctors and rheumatologists. If you have a patient with severe dry eye symptoms, it’s important to rule out Sjögren’s syndrome and other autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis. SS dry eye typically presents with significant and coalesced corneal (Figure 5) and conjunctival staining with sodium fluorescein and lissamine green.
Other autoimmune diseases such as fibromyalgia can exacerbate dry eye as well. A retrospective cohort study found that patients with fibromyalgia 49 years of age or older had an 80% elevated risk of dry eye syndrome compared with the non-fibromyalgia group.18 If a patient with fibromyalgia had a comorbidity of irritable bowel syndrome or sleep disturbance, the risk of dry eye increased even more.18
MasqueradersSuperior limbic keratoconjunctivitis may often be missed because this region is not examined as closely as the eyelashes, eyelids, MGs and the exposed interpalpebral conjunctiva and cornea. Microtrauma definitely occurs at this interface, described as frictional events in the TFOS DEWS II.20 This area, if compromised, will pick up vital dye staining and even appear hyperemic and overall inflamed. Therapies to reduce this inflammation as well lubricate these tissues against the blinking eyelid will ensure an expedient resolution of signs and symptoms. One should also rule out any thyroid dysfunction when coming across this specific ocular finding.19
Conjunctivochalasis is also a highly prevalent—greater than 90% in the fifth decade of life and onwards—and underdiagnosed condition that often presents with dry eye symptoms.25 Severe conjunctivochalasis may initially appear as an elevated tear meniscus height when in fact it is all conjunctival tissue contacting the inferior cornea. Tears may stagnate in the folds, worsen tear film stability and overall drain more slowly. Thus, the folds may also harbor inflammatory mediators as these frictional events perpetuate more symptoms and more inflammation.26 Initial therapies include strategies to reduce inflammation and promote lubrication across these micro or macro folds.26 Surgical treatment includes conjunctival cauterization or surgical excision with or without tissue graft. Radiofrequency cautery, similar to electrocoagulation, shortens conjunctiva by burning excessive conjunctiva at sites about 5mm from the limbus following topical anesthesia. These procedures are relatively short (less than 10 minutes) and can be performed in an outpatient clinic setting.27 The goal is to get the vast majority of the redundant conjunctiva to lay flat against the sclera and restore a smooth interpalpebral surface again, unimpeded by the blink. |
Another potential manifestation of fibromyalgia is neuropathic eye pain, a subtype of the TFOS DEWS II clinical decision algorithm that describes when a patient has dry eye symptoms without signs.2 One study found dry eye symptoms correlate with both neuropathic pain complaints, particularly the symptom of ocular burning, and the Patient Health Questionnaire-9, which is a measure of depression. In these cases, clinicians should refer to a pain specialist for treatment.13,14
Filamentary keratitis can also cause many symptoms secondary to frictional events and corneal nerve stimulation as the upper eyelid blinks and moves these filaments. Researchers found that filaments appeared in the exposed interpalpebral zone for dry eye and exposure keratitis, but were more likely at the corneal limbus for autoimmune and ocular inflammatory conditions.23 Filaments may present early as a single 0.25mm mucus collection on the corneal epithelium.7 After topical anesthesia, removing the filaments with a cotton-tipped applicator or forceps and applying a bandage soft contact lens dramatically improves symptoms. Continued daily wear of soft contact lenses also minimizes future formation of new filaments. Other treatments include nonpreserved lubricants, topical steroidal and nonsteroidal anti-inflammatory agents, hypertonic saline and mucolytic agents.24
Severe dry eye patients require frequent monitoring and treatment adjustments to ensure they achieve much-needed relief and corneal healing. While eye care providers should remain positive, they must also set realistic expectations to maintain, maximize what’s left and restore as much homeostasis as possible.
Dr. Kwan is an assistant professor at Southern California College of Optometry at Marshall B. Ketchum University. He is the current chief of the Stein Family Cornea and Contact Lens Center, University Eye Center, Ketchum Health. He is also director of the Dry Eye Institute for patient care and participates in dry eye and contact lens research.
|
1. Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94-101. |