In the past, stem cells were harvested primarily from cadavers. More recently, they came from autologous sources and live donations from relatives. But today, science has developed the capacity to cultivate limbal stem cells in vitro, and transplant them onto the recipient’s tissue bed in an effort to manage disease states.
This article provides an overview of these modern advancements.
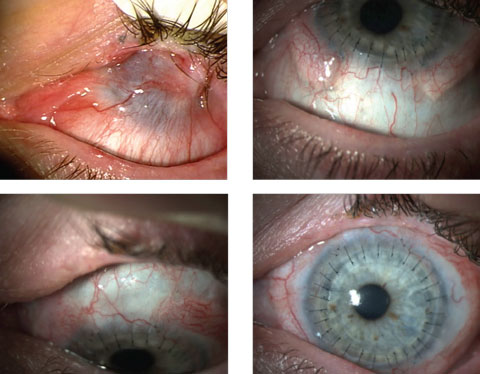 |
| These images show a patient with epithelial defects before (top left) and after stem cell therapy in conjunction with corneal transplantation. Click image to enlarge. Photos: Ed Holland, OD |
Pathophysiology
Corneal epithelial cells undergo constant mitosis and migration to provide a clear, smooth optical pathway for light to enter the eye and allow for visual stimulation.2-4While this basic process involves numerous levels of cellular activity, its primary responsibility—new cell development—is dependent upon a population of stem cells that reside in the palisades of Vogt at the limbal margin.2-4 These cells serve as an engine for cellular replication and corneal health.2-4 Additionally, the limbal margin serves as a barrier to infectious disease and a mediator for anterior segment inflammation.2-4 When violated, it can succumb to the progression of a disease, permitting it to enter corneal space.2-4
Corneal limbal stem cells, unlike other stem cells, are specifically directed toward developing new cellular tissue exactly like the originating cell.5 The lack of plasticity that is unique to corneal limbal stem cells differentiates them from other stem cell types. It is also what allows them to be relatively universal in their capacity to differentiate into a variety of tissues, including muscle cells, organ cells and vascular tissue.6
LCD Causes
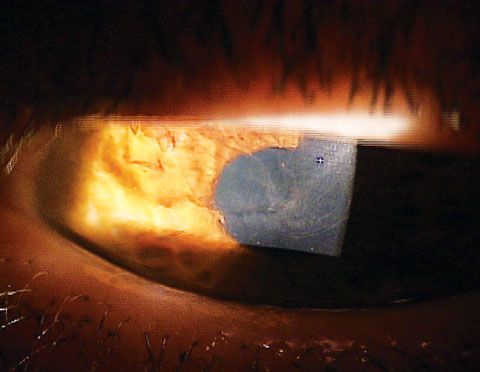
This LSCD patient could benefit from stem cell therapy. Etiologically, LSCD can derive from numerous clinical conditions. Inflammatory, infectious or traumatic sources can damage limbal cellular activity at a level which prevents normal re-establishment of physiology.4,8-11 In addition to chemical burns and Stevens-Johnson syndrome, other causes of LSCD include contact lens-induced keratitis, limbitis, aniridia, multiple ocular surgeries, peripheral ulcerative disorders, chronic neurotrophic keratitis, pterygium, severe microbial keratitis, chronic bullous keratopathy and radiation therapy. 4,8-11 |
On a Cellular Level
The stem cell network begins its differentiation during fetal development. Adult stem cells start out as embryonic stem cells—which are pluripotent—that is, capable of forming all cell lines and tissues necessary for the continued support to the body. In addition to the embryonic cells, science has now discovered techniques to revert adult stem cells back to a pluripotent state. These are known as induced pluripotent stem cells (iPSCs).1,5,7
Clinical Signs of Disease
Corneal limbal stem cell disease (LSCD) can be classified as either primary or secondary.12,13 Primary LSCD may be associated with conditions such as neurotrophic keratopathy, endocrine deficiencies and chronic inflammation.12,13 It typically does not have observable etiologic factors.12,13 Secondary LSCD occurs as a result of tissue destruction, such as in the case of contact lens wear, infectious disease (bacterial or viral), surgical trauma and thermal and ultraviolet injuries.12,13This can present with several clinical signs, such as:8-11
1. Irregular epithelial resurfacing that is specific to a segment of the cornea initiated at the limbus.
2. Sodium fluorescein, lissamine green and rose bengal staining of the cornea in a stippled and sometimes whorl-like irregular pattern.
3. The development of corneal erosions or ocular surface inflammatory disease with possible changes in matrix metalloproteinase-9 findings.
4. Corneal opacities.
5. Neovascularization.
Clinical signs and symptoms of LSCD typically include photophobia, injection, discomfort and pain.14 Neovascularization is frequently present in chronic cases typically associated with inflammation.14
While the diagnosis of corneal LSCD is usually confirmed by clinical signs and patient symptoms, doctors can also employ technologies such as impression cytology and histopathology to look for specific cellular changes relative to LSCD.14 Doctors can also biopsy tissue at the limbal margin to determine the underlying cause. Fluorophotometry looks at the dysfunction of the tissue at the limbal margin from a physiologic perspective.15
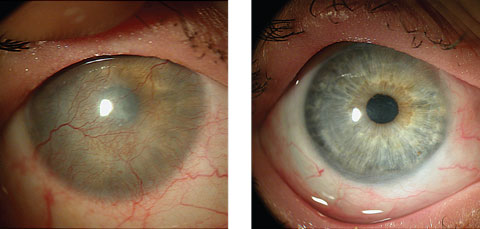 |
| At left, this patient’s LSCD was due to Stevens-Johnson syndrome. At right, a CLAL procedure (from her fellow eye) resulted in a healed, smooth corneal surface with no immunosuppression. Click image to enlarge. Photos: Scheffer Tseng, MD |
Treating Limbal Stem Cell Disease
Typically, clinicians don’t test for LSCD. More commonly, they treat the underlying issue and expect resolution. These treatments usually include cycloplegia and topical NSAIDs for analgesia, topical antibiotics to mitigate infective processes and steroids to mitigate inflammation. When these don’t work, clinicians can suspect LSCD. Research shows the use of topical steroids may be effective in mild cases.16-18 Low-dose topical steroids can be applied on a chronic basis, so long as appropriate follow-up is instituted to monitor for their possible complications. Research also shows other agents, such as cyclosporin A and Xiidra (lifitegrast, Shire), can be effective along with nonpreserved tears and ointments.16-18Mild LSCD. The typical rehabilitation timeline for patients with mild LSCD can be anywhere from weeks to months, or longer, and may need to be accompanied by debridement of the corneal epithelial tissue to remove the migrated conjunctival epithelial cells.16-18 This is best accomplished with a classic debridement technique and placement of an amniotic membrane to enhance surface healing and to encourage normal repopulation of the limbal margin.16-18
Once the epithelial surface has regenerated, the underlying cause resolved and the limbal margin returned to a normal population, the condition resolves.16-18
Severe LSCD. In patients with more serious presentations, limbal stem cell transplantation surgery can maximize outcomes. As a part of the preoperative assessment, review the periorbital tissue to assure that lid hygiene is optimal and both anterior and posterior blepharitis are treated. Clinicians also need to identify and treat abnormalities of lid position, such as ectropion and lagophthalmos.8,19,20
An additional risk includes glaucoma, due to the long-term need for topical steroid use in patients who have undergone limbal stem cell transplantation. Lotemax (loteprednol etabonate, Bausch + Lomb) in this setting is a reasonable treatment to avoid intraocular pressure (IOP) elevation, and is one that can be implemented to minimize risk of steroid-induced IOP rise. Toxicity from topical therapy (steroidal or anti-glaucoma) may decrease success in surgical intervention. This can be mitigated with the use of nonpreserved topical medications, selective laser trabeculoplasty or micropulse laser trabeculoplasty prior to initiating treatment. These procedures are designed to remove the need for chronic topical therapy or at the very least, decrease the dosage levels.
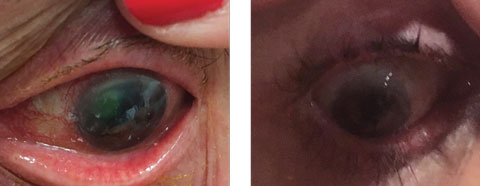 |
| This LSCD patient initially presented with 20/400 visual acuity with pinhole. Click image to enlarge. |
Surgical Limbal Stem Cell Transplantation
Limbal stem cell transplantation can be accomplished through several modalities.1,21,22 These include:1. Conjunctival limbal autograft (CLAU), which involves harvesting healthy donor tissue from an unaffected fellow healthy eye.
2. Conjunctival limbal allograft (CLAL), which uses a donor-matched tissue from a relative (typically indicated in cases of bilateral disease).
3. Keratolimbal allograft (KLAL), in which the tissue is acquired from a cadaver donor.
The method most commonly employed for either CLAU or CLAL involves the harvesting of conjunctival tissue progressing forward to the limbal margin and moving into clear cornea.23,24 This assures a full complement of limbal stem cells for transplantation. The tissue from the donor eye, or the healthy eye of the patient, is then transplanted to the recipient eye, which has been prepared by removing the damaged tissue to create a bed for the transplanted tissue.23,24 The use of fibrin glues to secure tissue is the most common method of wound closure.8
In conjunctival autograft harvesting, the donor eye could potentially develop LSCD as a result of the surgical manipulation of the tissue and removal of the limbal cellular bed.25 Identify any clinical complications to decrease trauma long-term to the donating eye, in addition to the treated eye, to enhance outcomes. Because the CLAU is a self-donated tissue, the patient faces no risk of immunologic rejection.25
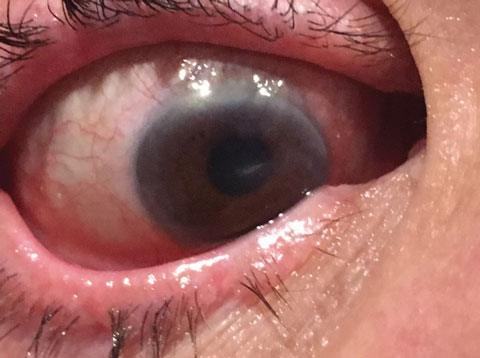 |
| This image revisits the patient from page 34. Two weeks after initiating limbal stem cell disease therapy, she was down to 20/100 with pinhole. |
Allograft Tissue Replacement
When disease states are bilateral, the use of an autograft is not possible. In this circumstance, an allograft from a living relative donor is an effective alternative to cadaveric tissue, studies show.19,20,26 Since allograft tissue has the same concerns as other donated tissue, the standards for excluding disease states, such as screening for hepatitis and HIV, needs to be implemented, and tissue which is not healthy should be excluded.19,20,26Allograft tissue replacement has the potential for rejection and requires topical and systemic immunosuppression therapy for long-term success. Because the procedure does not involve a complete 360-degree transplantation, it has limits in patients with a diffuse disease state (for example, burns or neurotrophic keratopathy). These patients are more likely to undergo cadaveric transplantation, while a living relative allograft is better suited for patients with specific zones of limbal stem cell damage.27-29
The third procedure, KLAL, which uses cadaveric tissue, was studied approximately two decades ago.30 Investigators have since evolved the technique to its current level, which requires the preparation of the recipient tissue antecedent to the placement of the corneo-scleral transplant tissue. The donor tissue is prepared through a complex surgical procedure that involves the dissection of both conjunctival, limbal and corneal tissue in preparation for the placement. After placement of the donated tissue, the use of a fibrin glue to secure the transplant, along with the positioning of an amniotic membrane to accelerate the regeneration of surgical wound site, is typical. The success rate varies considerably with this technique, but authors have found that upwards of three-fourths of patients were successful on long-term immunosuppressive therapy, with a variety of agents.30-32
Surgical intervention has been the primary method for addressing this complex problem, but new technology developed over the last decade may change that. The most recent advancement is the use of cultured corneal epithelial stem cells to replace the damaged tissue. This is accomplished with autologous stem cell systems, which eliminate the risk of rejection and the need for immunosuppressive therapy. We also have the ability to culture stem cells through generalized stem cell lines; however, because stem cell lines are specifically directed creates a greater level of difficulty for this technique. While this does not eliminate the need for anti-rejection therapy, nor does it completely block the body’s ability to reject the graft, it does present an exciting methodology.
The first series of cases in this area monitored patients with autologous cultured corneal epithelial stem cell transplantations over a two-year period, and described a relatively low rate of complications and notable visual improvement.31 In cases with more advanced disease states, the procedure was less successful, with less than 50% of the eyes achieving long-term stability without complications.32
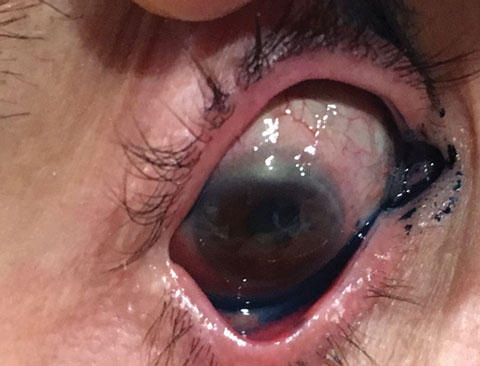 |
| Here is our patient again four weeks after her initial treatment. She is now at 20/40 with pinhole. |
Comanagement of LSCD
Initial treatment of surgical limbal stem cell disease patients is not unlike the therapy currently used for other transplanted ocular tissue. Patients are placed on a course of topical antibiotics, along with steroids, and can take additional anti-immune system management drugs, such as cyclosporin A. Clinicians should minimize the use of any and all preservative-based compounds when possible, along with surgical intervention to address abnormal lid positioning or other concerns. These agents can be used for significant lengths of time and may need to be indefinite for some patients to maintain optimized ocular surface status.33 This presents real risks relative to the development of secondary complications, such as secondary steroid-induced open-angle glaucoma and ocular surface-related issues in the form of toxicity, and can cause degradation of surgical outcomes.32 Rejection is always possible as both a short- and long-term concern. Depending on severity, oral immunosuppressants may need to be used adjunctively to topical therapy to minimize risk.33It is rare in a clinician’s lifetime to witness the birth of a new technology, but with today’s stem cell advancements, we stand at the dawn of a true paradigm shift. Welcome to the future.
Dr. Thimons is a founding partner with the Ophthalmic Consultants of Connecticut and an adjunct clinical professor at Salus University.
|
1. Fernandes M, Sangwan VS, Rao SK, et al. Limbal stem cell transplantation. Indian J Ophthalmol. 2004;52:5-22. 2. Secker G, Daniels J. Limbal epithelial stem cells of the cornea. In: StemBook (Online) 2009. Available: www.ncbi.nlm.nih.gov/books/NBK27054/. Accessed: January 24, 2017. 3. Beebe D, Masters B. Cell lineage and the differentiation of corneal epithelial cells. Invest Ophthalmol Vis Sci 1996;37:1815-25. 4. Chen JJ, Tseng SC. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:2219-33. 5. The Conversation of Africa. Stem cell research and therapy. Available: www.theconversation.com/a-beginners-guide-to-understanding-stem-cells-45502. Accessed: January 24, 2017. 6. Ebato B, Friend J, Thoft R. Comparison of limbal and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1988;29:1533-37. 7. American Society of Clinical Oncology. 2016. What is a stem cell transplant (bone marrow transplant)? Available: www.cancer.net/navigating-cancer-care/how-cancer-treated/bone-marrowstem-cell-transplantation. Accessed: January 24, 2017. 8. Bakhtiari P, Djalilian A. Update on limbal stem cell transplantation. Middle East Afr J Ophthalmol. 2010;17:9-14. 9. Dua HS, Joseph A, Shanmuganathan VA, Jones RE. Stem cell differentiation and the effects of deficiency. Eye. 2003;17:877-85. 10. Chen JJ, Tseng SC. Corneal wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1990;31:1301-14. 11. Vora GK, Daluvoy MB. Diagnosis and management of limbal stem cell deficiency. American Academy of Ophthalmology EyeNet. 2014;2:35-36. 12. Pajoohesh-Ganji A, Stepp MA. In search of markers for the stem cells of the corneal epithelium. Biol Cell. 2005;97:265-76. 13. Kinoshita S, Kiorpes TC, Friend J, Thft RA. Limbal epithelium in ocular surface wound healing. Invest Ophthalmol Vis Sci. 1982;23:73-80. 14. Puangsricharern V, Tseng SC. Cytologic evidence of corneal disease with limbal stem cell deficiency. Ophthalmology. 1995;102:1476-85. 15. Kinoshita S, Adachi W, Sotozono C, et al. Characteristics of human ocular surface epithelium. Prog Ret Eye Res. 2001;20:639-73. 16. Kim TC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473-84. 17. Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631-46. 18. Barash A, Narala R. 2010. A review of limbal stem cell deficiency including its etiology, pathophysiology, diagnosis and treatment. EyeWiki. Available: www.eyewiki.aao.org/Limbal_Stem_Cell_Deficiency. Accessed: January 24, 2017. 19. DeSousa JL, Daya S, Malhorta R. Adnexal surgery in patients undergoing ocular surface stem cell transplantation. Ophthalmology. 2009;116:235-42. 20. Korb DR, Greiner JV, Herman JP, et al. Lid-wiper epitheliopathy and dry-eye symptoms in contact lens wearers. CLAO J. 2002;28:211-6. 21. Shimazaki J, Higa K, Morito F, et al. Factors influencing outcomes in cultivated limbal epithelail transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143:945-53. 22. Stuart A, Holland EJ, Stenevi U, Watson S. Stem cells: anterior segment advances. American Academy of Ophthalmology EyeNet. 2012;12:25-7. 23. Tan D, Ficker L, Buckley R. Limbal transplantation. Ophthalmology. 1996;103:29-36. 24. Basti S, Rao SK. Current status of limbal conjunctival autograft. Curr Opin Ophthalmology. 2000;11:224-32. 25. Rao Sk, Rajagopal R, Sitalakshmi G, Padmanabhan P. Limbal autografting: comparison of results in the acute and chronic phases of ocular surface burns. Cornea. 1999;18:164-71. 26. He H, Yiu SC. Stem cell-based therapy for treating limbal stem cells deficiency: A review of different strategies. Saudi Journal of Ophthalmology. 2014;28:188-94. 27. Huang T, Wang Y, Zhang H, et al. Limbal allografting from living related donors to treat partial limbal deficiency secondary to ocular chemical burns. Arch Ophthalmol. 2011;129:1267-73. 28. Lam DS, Young AL, Leung AT, et al. Limbal stem cell allografting from related live donors for corneal surface reconstruction. Ophthalmology. 2000;107:411-12. 29. Tsubota K, Toda I, Saito H, et al. Reconstruction of corneal epithelium by limbal allograft transplantation for sever ocular surface disorders. Ophthalmology. 1995;102:1486-95. 30. Holland EJ, Djalilian AR, Schwartz GS. Management of Aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology. 2003;110:125-30. 31. Kim Jy, Djalilian AR, Schwartz GS, Holland EJ. Ocular surface reconstruction: Limbal stem cell transplantation. Ophthalmol Clin N Am. 2003;16:67-77. 32. Pellegrini G, Traverson CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990-3. 33. Holland EJ, Schwartz GS. The paton lecture ocular surface transplantation: 20 years’ experience. Cornea. 2004;23:425-31.1. |