I remember the day in the late 1970s that my mother came home from the hospital after donating her blood-creating bone marrow, aspirated from the iliac crests of her pelvis. My mother has a rare blood type and made the donation for a friend’s research thesis at the Memorial-Sloan Kettering Cancer Center (MSKCC) in New York City.

My mother, Frances Raab Mastrota, Ph.D., and father Dominick Joseph Mastrota. My mother, who has a rare blood type, donated bone marrow for groundbreaking
cancer research.
Renowned for cancer treatment and its groundbreaking cancer research, MSKCC has been a leader in bone marrow transplantation since its physicians performed the first successful transplant from an unrelated donor in 1973.1
I am proud to say my mother contributed to that process.
Why is bone marrow transplanted? Bone marrow contains somatic (adult) hematopoietic stem cells that can differentiate into all of the cell types contained in blood. Red blood cells, macrophages, monocytes, eosinophils, platelets, neutrophils, basophils and lymphocytes are all derived from the hematopoietic stem cell. Bone marrow (and more recently blood stem cells isolated from the peripheral blood stream) is transplanted routinely to treat a variety of blood and bone marrow diseases, blood cancers and immune disorders.
Stem Cells
What makes stem cells so special? Because of their capacity to self-renew and give rise to more mature progeny, stem cells are indispensable in maintaining the tissue integrity of multicellular organisms. More explicitly, they can generate daughter cells identical to their mother (self-renewal) and produce progeny with more restricted potential (differentiated cells).2
In mammals, embryonic stem cells are found in the inner cell mass of blastocysts, structures formed in early embryogenesis. These pluripotent cells have the potential to make any differentiated cell in the body. Various adult tissues in mammals also contain adult/somatic multipotent stem cells, which can differentiate into a limited number of types of cells.3 In adult organisms, stem cells regulate cell populations in tissues that undergo continuous turnover.4
In 2006, researchers learned how to genetically “reprogram” specialized (differentiated) adult murine cells to assume a stem cell-like state (induced pluripotent stem cells or iPSCs).5-7 The following year, iPSCs were established from human fibroblasts, marking a significant technological advance in cell-based regenerative/reparative medicine.8
Tissues derived from this new type of stem cell provide a nearly identical match to the cell donor, which prevents rejection by the immune system if transplanted into its donor.
Adult Stem Cells
Adult stem cell populations exist throughout the body. Besides the blood-forming hematopoietic stem cells that reside in the bone marrow, multipotent tissue-specific stem cell niches are found in the testes, the epidermis, hair follicles, the gut, dental pulp and the nervous system, among others.
During tissue regeneration, stem cells are induced to proliferate while maintaining an appropriate balance of self-renewal and differentiation divisions to sustain the stem cell pool and produce an adequate number of mature cells. The external signals for cell differentiation include chemicals secreted by other cells, physical contact with neighboring cells, and certain molecules in the microenvironment of the stem cell.9
Stem Cells and the Cornea
The corneal epithelium’s adult stem cell population is one of the better-understood stem cell systems in the body. The putative corneal stem cell population is believed to be localized to a specific niche in the limbal region.10-11 It is widely accepted that corneal stem cells can divide either symmetrically to produce two daughter stem cells or asymmetrically to create one daughter stem cell and one progenitor (transient amplifying) cell.
Transient amplifying cells, which have only limited proliferative capacity, migrate centripetally from the limbus and vertically from the basal epithelial layers forward, eventually giving rise to terminally differentiated cells.12-14
Terminally differentiated cells are highly specialized and have no proliferative capacity.15-16 The apical layers of the corneal epithelium are made of terminally differentiated cells. When the apical corneal epithelial cells reach the end of their life cycle, they are sloughed from the corneal surface and replaced by underlying maturing cells.
In the cornea, primary stem cell disease can occur in congenital disorders, such as aniridia, or as a manifestation of one of the ectodermal dysplasia syndromes. Secondary (acquired) stem cell disorders develop from external factors that damage stem cells with insult over time. Limbal stem cell deficiency can develop as a result of many conditions, including chemical, thermal or radiation injury, collagen-vascular diseases, medication toxicity, multiple ocular surgeries, and chronic ocular surface inflammation such as in rosacea, staph marginal disease, or chronic contact lens wear.
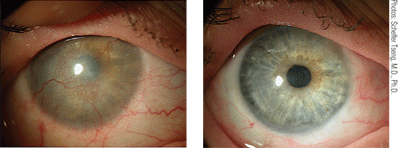
This patient presented with limbal stem cell deficiency due to Stevens-Johnson syndrome. Conjunctival limbal autograft from the fellow eye resulted in a healed, smooth corneal surface with no immunosuppression.
Restoration of corneal epithelial stem cell deficiency can be achieved by transplanting corneal stem cells. The first series of successful limbal stem cell transplants were published in 1989.17 In conjunctival limbal autografting, stem cell-containing limbal grafts are harvested from the patient’s healthy eye and transplanted into the stem-cell deficient eye. In comparison, conjunctival limbal allografting uses graft segments from living-relative eyes or even from cadaveric sources.
Stem Cells and Glaucoma
Glaucoma is a neurodegenerative disease that ultimately leads to retinal ganglion cell death. Research now suggests that neuroregeneration and repopulation of the visual pathway by stem cell or neural precursor cells is becoming possible. An increasing understanding of the pathways involved in directed axon growth and manipulation of stem cell and progenitor cells toward retinal ganglion cell fate have facilitated success in animal models of glaucoma.18
Additionally, local transplantation of stem cells has been demonstrated to be neuroprotective. A 2010 study documented that intravitreal injection of bone marrow stem cells protected optic nerve cells from further degeneration in a rat glaucoma model.19 Possible neuroprotective mechanisms offered by stem cell transplantation include the supply of neurotrophic support to injured axons and the modulation of matrix metalloproteases and other components of the central nervous system environment to facilitate endogenous repair.20
Interestingly, it has been suggested that a stem cell niche exists in the anterior trabecular meshwork beneath Schwalbe’s line.21 It may someday be possible to harvest and expand these trabecular meshwork stem cells ex vivo to replace missing or non-functional trabecular meshwork cells in glaucoma patients. This would prevent vision loss and alleviate the need for lasers, drugs and compliance, providing a lasting solution for these patients.
Stem Cells and the Retina
Although mammalian retinal stem cells have been isolated from the ciliary margin, renewal of retinal stem cells occurs to a very limited extent. These retinal stem cells, laboratory-stimulated RPE cells, neural stem cells and bone marrow stem cells are all sources for retinal cell-based therapies.
Multiple studies have demonstrated that transplanted stem cells are incorporated into the retina where the retinal microenvironment drives differentiation preferentially into photoreceptor cells and functionally rescue the retina.22
Recently, researchers for the first time have achieved recovery of vision in a rodent model of retinal dystrophy using embryonic stem cells differentiated into photoreceptors prior to transplant.23 In this study, mouse embryonic stem cells were induced to differentiate into RPE-like structures. After differentiation, these embryonic-derived cells were transplanted into the subretinal space of mouse retinitis pigmentosa models (mice in which a mutation of a rod-specific phosphodiesterase leads to rapid loss of photoreceptors during the early postnatal life).
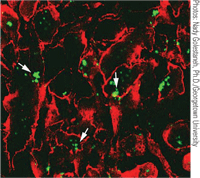
For the first time, human-induced pluripotent stem (hiPS) cells have been produced with RPE cell characteristics.
A quarter of the transplanted mice showed increased electroretingram response, suggesting improved visual function. Significant complications after transplantation in these experiments included retinal detachment or tumor formation. Nevertheless, this study demonstrated that differentiated embryonic stem cells have the potential to develop new therapeutic approaches for RPE-specific diseases, such as retinitis pigmentosa and macular degeneration.23-24
Human embryonic stem cells have also been coaxed into developing a three-dimensional retina construct, consisting of retinal pigment epithelium and early retinal progenitor cells.25 While still in the very early stages of discovery, embryonic stem cell-derived retinal tissue may help treat loss of vision due to retinal disease.
Researchers have demonstrated that mouse fibroblast iPSCs generated a wide range of retinal cell types, including retinal ganglion cells, cone and rod photoreceptors.26 In March 2011, other researchers demonstrated the ability to create retinal cells derived from human iPSCs.27
The retinal potential of iPSCs suggests that they may be used to formulate stem cell approaches to understand and treat a wide range of retinal degenerative diseases—from glaucoma to age-related macular degeneration.
The Future of Regenerative Medicine
Pioneering research shows that stem cell transplantation is technically feasible and provides tantalizing evidence for new possibilities to address otherwise untreatable blindness secondary to corneal, glaucomatous and retinal disease. Stem cell science, regenerative medicine and cell therapies are all part of this emerging paradigm shift in the treatment of ocular and other diseases.
In October 2011, the World Stem Cell Summit will bring together scientists, patients, advocates, business people, investors, educators, ethicists, policy makers, government representatives and others to share their knowledge with one another. Its goal is to create a meeting that delivers compelling content to facilitate, accelerate and translate groundbreaking biomedical research into vision and life-saving treatments and cures.28
Dr. Mastrota is the Center Director of Omni Eye Surgery in New York City.
1. Memorial Sloan-Kettering Cancer Center. Available at:
www.mskcc.org/mskcc/html/89568.cfm. Accessed March 14, 2011.
2. Melton DA, Cowen C. “Stemness”: definitions, criteria, and standards. In: Lanza R, Gearhart J, Hogan B, et al., eds. Essentials of Stem Cell Biology. 2nd ed. San Diego, CA: Academic Press; 2009: xxiii.
3. Barrilleaux, B, Phinney DG, Prockop DJ, O’Connor KC. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006 Nov;12(11):3007-19.
4. Fuchs E, Segre JA. Stem cells: a new lease on life. Cell. 2000 Jan;100(1):143-155.
5. Nakagawa M, Yamanaka S. Reprogramming of somatic cells to pluripotency. Adv Exp Med Biol. 2010;695:215-24.
6. Liu SP, Fu RH, Huang YC, et al. Induced pluripotent stem (iPS) cell research overview. Cell Transplant. 2011;20(1):15-9. Epub 2010 Sep 30.
7. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug;126(4):663-76. Epub 2006 Aug 10.
8. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov;131(5):861-72.
9. National Institutes of Health, U.S. Department of Health and Human Services. Stem Cell Basics: What are the unique properties of all stem cells? Stem Cell Information. Available at: http://
stemcells.nih.gov/info/basics/basics2.asp (accessed April 4, 2011).
10. Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004 Mar;78(3):433-46.
11. Dua HS, Shanmuganathan VA, Powell-Richards AO, et al. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005 May;89(5):529-32.
12. Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci.1983 Oct;24(1):1442-3.
13. Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci.1998 Oct;111(Pt 19):2867-75.
14. Dua HS, Gomes JA, Singh A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78(5): 401-8.
15. Sun TT, Lavker RM. Corneal epithelia cells: past, present and future. J Investig Dermatol Symp Proc. 2004 Sep;9(3):202-7
16. German MJ, Pollack HM, Zhao B, et al. Characterization of putative stem cell populations in the cornea using synchrotron infrared microspectroscopy. Invest Ophthalmol Vis Sci. 2006 Jun;47(6):2417-21.
17. Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthamology. 1989 May;96(5):709-23.
18. Dahlmann-Noor AH, Vijay S, Limb GA, Kwah PT. Strategies for optic nerve rescue and regeneration in glaucoma and other optic neuropathies. Drug Discov Today. 2010 Apr;15(7-8):287-99. Epub 2010 Mar 1.
19. Johnson TV, Bull ND, Hunt DP, et al. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010 Apr;51(4):2051-9.
20. Bull ND, Johnson TV, Martin KR. Stem cells for neuroprotection in glaucoma. Prog Brain Res. 2008;173:511-9.
21. Kelley MJ, Rose AY, Keller KE, et al. Stem cells in the trabecular meshwork: present and future promises. Exp Eye Res. 2009 Apr;88(4):747-51.
22. Stern J, Radosevich M, Temple S. Stem cell retinal replacement therapy. World Stem Cell Report 2009. Available at:
www.scribd.com/doc/26800847/World-Stem-Cell-Summit-Retinal-Replacement-Therapy. Accessed April 6, 2011.
23. Wang NK, Tosi j, Kasanuki JM, et al. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa. Transplantation. 2010 Apr;89(8):911-9.
24. Dahlmann-Noor A, Vijay S, Jayaram H. Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Can J Ophthalmol. 2010 Aug;45(4):333-41.
25. Nistor G, Seiler MJ, Yan F, et al. Three-dimensional early retinal progenitor 3D tissue derived from human embryonic stem cells. J Neurosci Methods. 2010 Jun;190(1):63-70.
26. Parameswaran S, Balasubramanian S, Babai N. Induced pluripotent stem cells generate both retinal ganglion cells and photoreceptors: therapeutic implications in degenerative changes in glaucoma and age-related macular degeneration. Stem Cells. 2010 Apr;28(4):695-703.
27. Kokkinaki M, Sahibzada N, Golestaneh N. Human iPS-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized VEGF secretion and gene expression pattern similar to native RPE. Stem Cells. 2011 Mar 24 [Epub ahead of print]. doi:10.1002/stem.635
28. World Stem Cell Summit. Available at:
www.worldstemcellsummit.com. Accessed April 6, 2011.

