|
Q: I have a few patients with a keratoprosthesis (KPro). What does this procedure involve? What form of prophylaxis is recommended to reduce risk of complications?
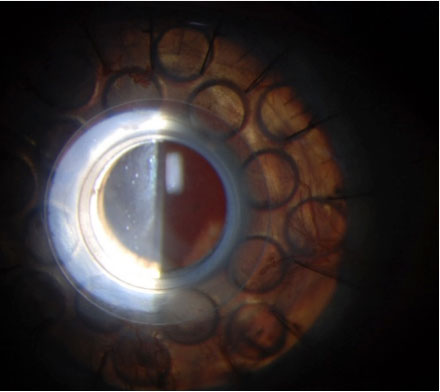
A: “The most common artificial cornea used today is the Boston KPro,” says Larae Zimprich, OD, of Vance Thompson Vision. A KPro is indicated in poor candidates for PKP due to reasons such as repeated graft failure, aniridia, Stevens-Johnson syndrome, chemical burn and autoimmune disease, in which there is often complete or nearly complete stem cell deficiency.
Since FDA approval in 1992, more than 12,000 patients have been implanting with a KPro.1 The most widely used (Type I) is for patients with adequate or normal tear function.2 Type II is reserved for end-stage corneal disease and requires a permanent tarsorrhaphy.2
The KPro Type I consists of a clear PMMA front plate, a back plate of titanium or PMMA that contains 16 fenestrations to allow passage of nutrients, and a locking ring behind the plates to secure the system.2 The corneal graft is situated between the two plates.2
|
|
The Procedure
KPro insertion typically takes 1.5 hours. Patients are monitored under general anesthesia or a retrobulbar block.3 First, a surgeon marks the center of the host cornea by using a Sinskey hook and measures the cornea to determine the appropriate transplant size. Then, the KPro is assembled. A donor cornea with a 3mm central hole is sandwiched between the front and back plates. The locking ring binds the system together.
Next, the surgeon trephines the recipient’s cornea. A paracentesis is created for injection of viscoelastic into the anterior chamber to maintain structure and stability. The KPro is then sutured into place with at least 12 uninterrupted or running 9-0 nylon sutures. Once the sutures are buried and Seidel-negative, a bandage contact lens is placed on the eye. Following surgery, an antibiotic and a steroid are injected into the conjunctiva.2
Post-surgical complications such as retroprosthetic membranes, glaucoma, cataracts, infection, retinal detachment, device extrusion and stromal necrosis can occur. For this reason, other procedures can be combined with the KPro, such as cataract removal or IOP-lowering device implantation. Educate patients on the risk of adverse effects and the associated prognosis.
Follow-up
Long-term care by different specialists, including cornea, glaucoma and retina, is required for patients with a KPro, according to Dr. Zimprich. One of the most common complications is endophthalmitis. To date, the overall incidence of endophthalmitis is 2.7%, which is 67.5 times greater than the rate associated with cataract surgery.2 To decrease endophthalmitis risk, a cocktail of medications is typically administered during surgery and continued prophylactically over a patient’s lifetime.
While there’s controversy over which antibiotic should be taken long-term, a broad-spectrum, fourth-gen fluoroquinolone (moxifloxacin or gatifloxacin) is most commonly used, says Dr. Zimprich. In autoimmune or monocular patients, fortified vancomycin is prescribed. These should be taken QD.4
Following surgery, patients require long-term bandage contact lens wear to prevent surface dehydration and enhance graft retention, notes Dr. Zimprich. Consequently, fungal keratitis is more likely to occur. Prescribe patients amphotericin B (0.15%) or natamycin (5%) to use as needed.4 Lens removal and replacement every three months or so is crucial.4 Betadine is recommended before lens reinsertion.
Lifelong topical steroids (e.g., prednisolone acetate) are required in all KPro eyes, says Dr. Zimprich. After the initial post-op period, taper down to once daily.4 Close monitoring is necessary, as steroids can exacerbate infection. If irritation occurs, patients should stop steroid use and see an eye specialist immediately.
Repeat all glaucoma tests every six months, suggests Dr. Zimprich. The central cornea is no longer intact; therefore, measure IOP every three months using a tactile approach. This is why many surgeons implant an IOP-lowering device in glaucoma suspects.4
Dr. Shovlin, a senior optometrist at Northeastern Eye Institute in Scranton, PA, is a fellow and past president of the American Academy of Optometry and a clinical editor of Review of Optometry and Review of Cornea & Contact Lenses. He consults for Kala, Aerie, AbbVie, Novartis, Hubble and Bausch + Lomb and is on the medical advisory panel for Lentechs.
1. KPro Study Group. kpro.org. Accessed March 19, 2021. 2. Boston keratoprosthesis (KPro). EyeWiki. eyewiki.aao.org/Boston_Keratoprosthesis_(KPro). Accessed March 19, 2021. 3. Keratoprosthesis patient management. Mass Eye and Ear. www.masseyeandear.org/medical-professionals/keratoprosthesis. Accessed March 19, 2021. 4. Recommendations: Boston KPro follow-up. Harvard Medical School. eye.hms.harvard.edu/eyeinsights/2015-september/recommendations-for-boston-kpro-follow-up. Accessed March 19, 2021. |