 |
A 69-year-old white female presented to the office for an evaluation in June with complaints of visual blur at distance and near. She reported that her vision had changed gradually in the previous six months and relatively symmetrically between the eyes. Prior to entering the room to evaluate the patient, I glanced at her chart and noticed a routing sheet from a visit three years earlier, at which point she was scheduled for a glaucoma progression evaluation, to which she never showed.
She confirmed the history obtained by my technician that her distance and near vision had gradually changed and that she felt she needed an update to her eyeglass prescription. I prodded her a bit about our last visit and the discussion we had about her being a very strong glaucoma suspect. She implied that she had no real recollection of that conversation, though she admitted that I had said she needed to return shortly after having been seen, but since her eyes “weren’t bothering” her, she didn’t feel it was necessary.
Diagnostic Data
Her systemic medications included Prilosec (omeprazole, Procter & Gamble) and Vytorin (ezetimibe/simvastatin, Merck) and she reported no allergies to medications. She mentioned that she was prediabetic and, on further probing, I got the impression that her PCP had noted a gradual increase in her A1c and discussed with her the possibility that she may be headed toward medication.
Her entering visual acuities were 20/50 OD and 20/40 OS through hyperopic astigmatic correction. Best-corrected acuities were 20/20 OD and OS and 20/20 OU through an increased hyperopic and astigmatic correction. Pupils were equal, round and responsive to light and accommodation with no afferent pupillary defect. Extraocular movements were full in all positions of gaze.
 |
| Fig. 1. Close examination of the TSNIT graph in this OCT scan shows a loss of RNFL in the superior temporal sector, in the location marked, of 51µm. Close examination of the optic nerve image shows a subtle but distinctly present wedge defect. |
A slit lamp examination of her anterior segments demonstrated clear corneas, slightly narrowed angles by Van Herick estimation and a quiet anterior chamber in both eyes. Applanation tensions were 29mm Hg OD and 31mm Hg OS at 10:55am. IOPs at her last visit were 26mm Hg OD and OS at 3:00pm. She was dilated in the usual fashion with phenylephrine and tropicamide. Pachymetry readings obtained at the previous visit were 513µm OD and 509µm OS.
Examination of her crystalline lenses was characterized by early nuclear cataracts with cortical spokes in both eyes. Previously, she was noted to have incipient lens changes consistent with her age. The vitreous examination was essentially unremarkable, except for vitreous syneresis. Her cup-to-disc ratios were estimated to be 0.55 x 0.65 OD and 0.55 x 0.75 OS. There was a subtle RNFL wedge defect noted superior temporally in her left eye, which had not been documented previously. Her retinal vascular evaluation was consistent with mild arteriolarsclerotic retinopathy in both eyes. Her macular evaluations were remarkable only for fine RPE granulation, consistent with her age. Her peripheral retinal evaluations were unremarkable.
Given that she was already dilated, we took optic nerve photos and obtained both HRT 3 optic nerve scans, as well as OCT imaging of the RNFL and the macular region in both eyes. Close examination of the disc photos demonstrated minimal change, as the original images were not of the best quality. HRT 3 imaging showed a subtle difference in the neuroretinal rims in the right and left eyes and was consistent with her estimated cup-to-disc ratio. OCT evaluations demonstrated several interesting findings worthy of further discussion, including a focal loss of the perioptic RNFL in the superotemporal (ST) sector of the left eye, consistent with the fundus findings.
Findings
Compliance (or lack thereof) aside, this case demonstrates the classic findings associated with glaucomatous optic neuropathy as it progresses. Certainly, compliance will need to be addressed, and a treatment plan established that will be conducive to patient compliance. But let’s take a look at several important findings in the OCT imaging of her left eye.
 |
| Fig. 2. The image in the lower right shows a decline in the ganglion cell layer in the superior arcuate region. |
We can see the standard RNFL circle scan common to all OCT instruments in the context of glaucoma, along with the TSNIT graphs (Figure 1). The images also show the current RNFL scan overlying the baseline RNFL scan obtained three years earlier. Close examination of the TSNIT graph shows a clear and focal loss of RNFL in the ST sector, in the location marked, of 51µm. Close examination of the optic nerve image in the same figure, at the area under observation, shows the subtle but distinctly present wedge defect extending outward. However, many clinicians tend to gravitate their attention to the statistical database comparisons, looking for a quick reference of whether the patient falls within (green), borderline (yellow) or outside (red) normal limits.
Looking at this same scan, in particular at the Garway-Heath sectors, one sees that the parameters of the sector analysis (all green) do fall within normal limits. If not closely examining the details of this scan, one might assume that everything is, in fact, fine, when it truly isn’t.
And herein lies one of the shortfalls of normative databases: they are simply statistical measures of where your patient fits into the normal bell curve distribution of patient parameters. Why are the sectors green if there is a wedge defect and there is a noted TSNIT change? The answer is simple: the TSNIT is looking at each point along the scan reference line, whereas the Garway-Heath sectors are looking at the overall (global) sections of the optic nerve. While there is a decrease in the RNFL in the ST focal area, that change is not enough to statistically alter the Garway-Heath sector analysis; thus each sector, including the ST sector, remains green. I’ve mentioned on numerous occasions previously the presence of “red disease.” Well, this is just the opposite: green wellness.
OCT readings show the ganglion cell layer scans of the macular region in her left eye, with the baseline image from 2013 at the top, and the current scan in the middle (Figure 2). The image in the lower right clearly shows a decline in the ganglion cell layer (not ganglion cell complex) in the superior arcuate region—consistent with the RNFL wedge defect seen clinically and on the circle OCT scan—and extending to the temporal horizontal raphe. Not surprisingly, this structural defect produces an arcuate visual field defect inferiorly, with a nasal step, obtained with field testing at a date subsequent to this image.
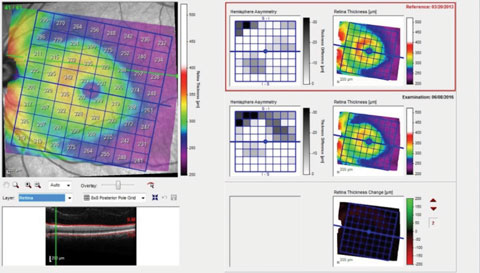 |
| Fig. 3. This OCT scan shows the patient’s overall macular retinal thickness. |
Finally, Figure 3 shows a similar printout but of the overall macular retinal thickness, rather than just the ganglion cell layer, as in Figure 2. Note here that the retinal thickness change demonstrates negligible difference between the two visits, whereas in Figure 2, the ganglion cell layer change was readily identifiable. Again, the explanation is simple: detecting a few microns difference in tissue (total retina) that is 300µm thick vs. a few microns difference in tissue (ganglion cell layer) that is 50µm thick, is proportionally less noticeable. So when we look at the retinal thickness change analysis and see no difference, the tendency might be to assume that there is no substantive difference.
The important point here is that we as clinicians need to be intimately aware of what exactly our instruments of choice are showing us, and consequently, what is the clinical significance, if any, of that information. While the hemisphere asymmetry reports in Figure 3 show change, the retinal thickness change color map does not. Interestingly, the hemisphere asymmetry report in Figure 3 correlates well with the ganglion cell thickness change report in Figure 2, owing to the loss of ganglion cells in this area.
Encouraging Compliance
So, what does all this mean, other than she got worse? As mentioned above, it means we all need to step back from our instruments for a few minutes and look carefully at the information those instruments are giving us. What exactly does this information mean, in the context of the patient in front of us? As we begin to talk more and more about personalized glaucoma care, we need to realize that the most significant player in allowing us to provide the personalized care is not this instrument or that instrument; rather, it is our clinical decision making process that can drive us to provide that personalized care every patient deserves.
Now, getting back to the patient at hand and her management: clearly she has glaucoma that has worsened, and she has been noncompliant in the past. How should you proceed? There are several viable options.
The advantage of SLT therapy is evident on two counts. First, SLT tends to be more effective on eyes that are not chronically medicated, and can result in lower IOP than in eyes already medicated. And second, if she continues to be noncompliant, perhaps the SLT will offer her some protection from herself.
Alternatively, medical therapy is also an option, and can possibly result in adequate IOP reduction with a fixed combination drug. But that option presupposes her being compliant. Compliance can be difficult to obtain. The simple truth is that some patients are more compliant than others. The recalcitrant noncomplier is difficult to deal with, and probably should be dismissed from your practice. But in cases like this, where the patient claims to not have known the significance of her condition, maybe we should start with modifying what we say and do to help foster that desired compliance by getting the patient to buy in to their own care. I would certainly rather have a patient who is aware of the need for compliance than simply just being indifferent to me and their condition.
Either way one proceeds (SLT or medical therapy), the patient will still need to be seen for progress evaluations to determine stability. If she complies with those evaluations, there’s a good chance she can be stabilized; if not, then she probably will get worse. A patient always has to assume a certain amount of responsibility for their own care.

