 |
For many years, blepharitis and dry eye disease (DED) were considered two completely independent diseases. But the Rynerson theory of dry eye blepharitis syndrome (DEBS), recently published in Clinical Ophthalmology, suggests dry eye may be the result of decades of chronic blepharitis.1 Let’s take a closer look at what this might mean for clinical practice.
The Biofilm Bridge
We’ve heard a lot about biofilms lately, especially with overused contact lens cases, for example. Although a biofilm can accumulate on an inert structure such as a stent or contact lens case, it can also exist on a living structure.2 For instance, plaque on your teeth is essentially biofilm formation.3
Depending on the age of the patient, contact lens use, Demodex and other factors, various manifestations of blepharitis may demonstrate various degrees of lid margin “scurf” or debris—biofilm. But the key factor is inflammation, and if it exists, blepharitis is present and should probably be considered as the underlying disease process.1
The diagnosis of blepharitis and DED has been difficult in the past because both of these conditions have significant overlap, hence the theory of causation. As an example, multiple symptoms of blepharitits can overlap with those of DED, ranging from dry, gritty, irritated and itching eyes to tearing and blurred vision.4 In addition to multiple similar symptoms, both conditions can be slowly progressive and chronic with various manifestations depending on the stage of the disease.4,5 However, examining the eyelid margins more closely for biofilm formation may serve as a bridge of understanding between these two poorly understood diseases.
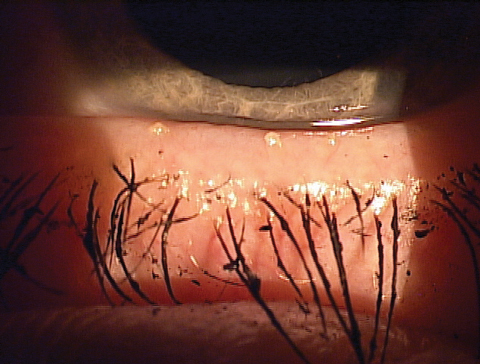 |
| Expression showing thickened meibum and the ‘volcano’ sign along the lashes. |
From Lid Margin Disease to DED
According to the new theory, lid margin disease progresses to inflammation through six steps: (1) bacterial survival, (2) biofilm formation, (3) over-colonization, (4) quorum-sensing gene activation, (5) virulence factor production and (6) inflammation that affects the lash follicles, meibomian glands and lacrimal glands.1 To begin the process, bacteria must survive enzymes such as lactoferrin, tear flow, natural cleaning activities of the eyelids with each blink and the protective mechanism of mucin secreted by goblet cells.6 When a patient’s blink rate decreases due to surgery, extensive digital device use, the use of eye drops containing preservatives, systemic medications that decrease tear volume, presence of various comorbidities and a host of multifactorial contributors, bacteria can survive longer.
Next is biofilm formation, described as “the prevailing microbial lifestyle.”7,8 Biofilm formation is a survival tactic allowing the bacteria to avoid desiccation and host responses, produce virulence facts and communicate with other bacterial species (as quorum-sensing). Biofilm adherence exists in many bacteria, but Staphylococcus in particular produces a protein known as adhesin that ensures a tight adherence to the surface.9 The lid margin is a common a site of adherence, considering one study shows 32 of the isolates cultured from eyes immediately after cataract surgery had the ability to form biofilms.10
Furthermore, although we wash and shower often, we don’t naturally wash or clean our eyelid margins—particularly the inner eyelid margins. In fact, most people tightly close their eyes when washing their face to prevent access to the eyelid margins. This leads to the slow, progressive, chronic destruction that occurs via inflammation over decades, eventually resulting in dry eye and even damage to the lid structure itself.
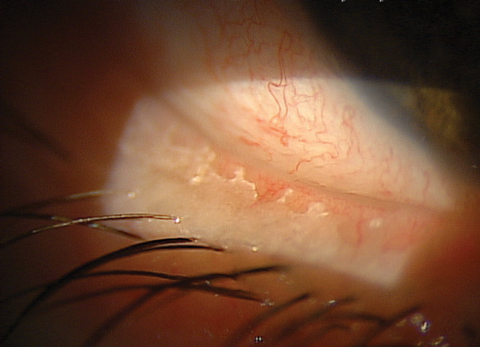 |
| You can see significant biofilm on this patient’s lid margin, a common site for biofilm adherence. |
The Four Stages of DEBS
The Rynerson theory also suggests stages of DEBS progression.
Stage 1 involves the lash follicles, where a biofilm can establish itself. This can often be assessed under high magnification for a ‘volcano sign’ when the base of the lash appears edematous. Scurf, or cylindrical dandruff in cases of Demodex blepharitis, is a sign of progression; however, these descriptions are misnomers and likely represent biofilm that has accumulated around the lash that pulled off as the lash grew.1
Stage 2 DEBS involves both the lash follicles and the meibomian glands and may explain obvious vs. non-obvious meibominan gland dysfunction (MGD). Because the biofilm blocks the large meibomian gland orifices (a combination of biofilm and poor or altered meibum), stage 2 takes longer to achieve.1
Stage 3 involves the follicles, meibomian glands and the accessory lacrimal glands of Krause and Wolfring. The distance, narrow ducts and constant tear flushing serve to protect these glands for decades, making them the last glands affected by biofilm formation.1
Stage 4 occurs when the structural integrity of the eyelid finally breaks down due to the chronic inflammation, which can manifest clinically as lid laxity, floppy eyelid syndrome, ectropion and entropion, for example.11,12
This DEBS theory may help explain any number of factors, including how bacteria can survive a Betadine prep prior to surgical procedures, resulting in endophthalmitis—the bacteria is at the stage of biofilm formation when it is resistant to an antiseptic.10
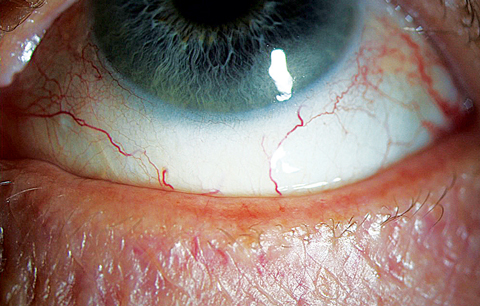 |
| This 65-year-old woman presented with frequent dryness and irritation, and she reported a “dry eye diagnosis” from a previous practitioner. A closer look at her lid margin suggests blepharitis is at play here as well. Click image to enlarge. |
Putting Theory Into Practice
I can’t prove or disprove this theory, but I have seen a significant positive impact when treating the biofilm mechanically for my DED patients, in addition to anti-inflammatory treatment and managing the obstructed meibomian glands. Furthermore, mechanical removal of the biofilm from the lid margin shows a profound impact on symptoms, quality of tears and quality of life.13
This theory affects so much of the optometric practice from a pathology perspective, including contact lens wearers who are more prone to early MGD/blepharitis and DED.14-16 With this knowledge, we may help patients remain in contact lenses longer by consciously focusing on and managing the biofilm component of ocular surface disease. Dentistry has mastered this important aspect of prevention by addressed biofilm formation with regular cleanings, brushing and flossing; this new theory suggests a similar model that could be even more critical in eye care.
Dr. Karpecki is a consultant to Blephex, OcuSoft, Bruder Healthcare, Paragon Biotech, TearScience and Akorn.
1. Rynerson JM, Perry HD. DEBS–a unification theory for dry eye and blepharitis. Clin Ophthalmol. 2016;10: 2455–67. |

