When differentiating episcleritis from scleritis, clinicians often use the phenylephrine blanching technique: blanching congested conjunctival and superficial episcleral blood vessels with either the 2.5% or the 10% concentration.1-4 When the deep episcleral plexus does not blanch, the diagnosis is usually scleritis. If the redness does disappear, it’s episcleritis.
But sometimes the answer is not that simple. For example, overlapping clinical features or variations in perceived patient pain may cloud the decision-making process. But a prompt and precise diagnosis is critical, as treatment and potential sequelae differ between the two clinical presentations.5 Here, we discuss the differences between episcleritis and scleritis—and how you can identify each of them accurately.
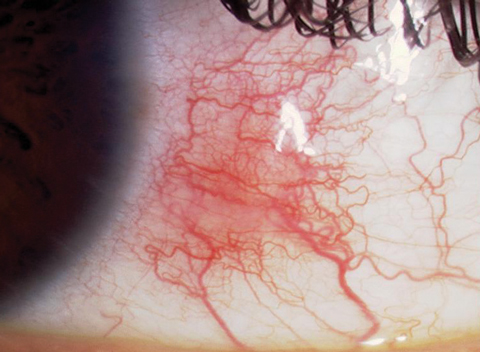 |
| Simple episcleritis is the most common presentation.1,11 |
Episcleritis
The episclera, the outermost layer, is composed of loose connective tissue with two vascular plexi (superficial and deep) derived from the anterior ciliary arteries.6 Normally, these vessels—which run forward from the insertions of the recti muscles—are not visible because they run deep to the conjunctiva. However, inflammation can make them observable, and the superficial plexus anastomoses with the conjunctival and deep episcleral plexus at the limbus. The superficial vessels are mobile, while the deeper ones are more firmly attached to the sclera.7
Episcleritis, also known as subconjunctivitis, phlegmatous conjunctivitis and episcleritis periodica fugax, is a benign inflammation of the conjunctival and superficial episcleral vascular plexi.8,9 Simple episcleritis is diffuse inflammation, while nodular episcleritis indicates a localized process with a well-defined area of elevation.1,11
Episcleritis is often a self-limiting condition with a proposed incidence of 21.7 per 100,000 person-years.12-14 While rare in children, roughly two-thirds of cases occur in females with a peak incidence in the fourth decade.1,2,8,15
Scleritis
The sclera consists of a collagen scaffold, glycoproteins, proteoglycans and protein fibrils.2,9 It is anteriorly contiguous with the cornea and posteriorly with the optic nerve’s dural and arachnoid sheaths. The sclera is weakened in this area due to perforations by the axons of the optic nerve (lamina cribrosa).9 This anatomical relationship explains why optic nerve edema may occur with inflammation of the surrounding posterior sclera.
Although the sclera is essentially avascular, it does have a rich supply of sensory nerves.6 Nutrient delivery comes from the choroid and episcleral vacular complexes.2,16 This, combined with the sclera’s web-like organization, slows the removal of antigens and other materials, providing an ideal environment for persistent inflammation.2
Normally, the fibrous sclera appears opaque, but in children it may look bluish due to the visibility of the underlying choroid. As patients age, it may appear more yellow due to fat deposition. The sclera measures roughly 0.45mm at the recti insertions and is thickest at the posterior pole (1.1mm to 1.3mm), which is important to remember when assessing for posterior scleritis using B-scan ultrasonography.17
Scleritis is separated into anterior or posterior, based on the inflammation’s location in relation to the extraocular muscle insertion sites.3,11,18 Anterior scleritis is further categorized into diffuse, nodular and necrotizing forms.11 Necrotizing scleritis may result in scleral perforation, which can occur without inflammation and is termed scleromalacia perforans.18
Most practitioners judge the severity of scleritis on a numerical scale based on their clinical experience. However, researchers fostered an eight-component, subjective scoring scale to grade scleritis.19 Because this scale did not assess inflammation, others established a system of standardized images for grading.20 While helpful, limitations to this study include the instillation of 10% phenylephrine up to 20 minutes prior to photography and the documentation of only one quadrant.20
Scleritis, however, is considered a chronic inflammatory response that involves the superficial and deep episcleral plexus.9,21 The exact mechanism of inflammation in scleritis remains unclear, as some report a variety of immunopathological findings, while others point to granulomatous inflammation and collagen disruption, which can lead to loss of tissue and subsequent thinning.8,9,16,21
Scleritis has a proposed incidence of 4.1 cases per 100,000 person-years and can be idiopathic, associated with a systemic autoimmune disease, surgically induced or infectious, although only 4% to 10% of all cases are deemed infectious.2,14,22 Potential pathogens include bacteria, fungi, parasites and viruses.
Ocular surgery and trauma account for almost half of all bacterial infectious scleritis, and most others result secondarily from a severe corneal infection.21,23 Pterygium excision and scleral buckling represent 75% of surgical causes, and Pseudomonas aeruginosa is the most likely causative agent.2,9,24 Researchers speculate tissue and blood vessel destruction during ophthalmic procedures may increase susceptibility to infection and explain the late onset of scleritis.9,24 Fungal and parasitic etiologies are rare.
The most common infectious cause overall is the herpes zoster virus (HZV), which is consistent with the greater-than-fourfold increase in HZV incidence over the last 60 years.22,25 Though uncommon, scleritis has been reported with bisphosphonate use—a class of drugs used to treat osteoporosis.12,21
Many ocular procedures may trigger surgically-induced necrotizing scleritis (SINS)—most commonly after limbal-incision cataract surgery—and 75% of patients who develop SINS have undergone two or more procedures.9
Table 1. Systemic Disease Workup | |
| Diagnostic Test | Remarks |
| Complete blood count | Elevated white cell count in infections |
| Basic metabolic panel | Evaluate for vasculitis-related renal disease |
| Erythrocyte sedimentation rate | Nonspecific inflammation |
| C-reactive protein | Nonspecific inflammation, acute |
| HLA B27 | Possible posterior scleritis association36 |
| Antineutrophil cytoplasmic antibodies | Granulomatosis with polyangiitis |
| Rheumatoid factor | Rheumatoid arthritis |
| Anti-cyclic citrullinated peptide | Prognostic indicator for RA severity |
| Antinuclear antibody | Systemic lupus erythematosus |
| Angiotensin-converting enzyme | Sarcoidosis |
| Chest x-ray | Sarcoidosis |
| Fluorescent treponemal antibody absorption | Syphilis |
| Rapid plasma reagin | Syphilis |
| Tuberculosis testing | Mantoux skin or Quantiferon Gold blood |
| Lyme serology | Lyme disease |
Symptoms
Here are the symptoms to look out for that can help you differentiate between episcleritis and scleritis:
Episcleritis. Generally, this does not result in significant pain, and usually patients only complain of mild discomfort or irritation.4,12 Episcleritis may present with epiphora but does not result in decreased acuity.2,3,12
Scleritis. This has the potential for sight-threatening sequelae.26,27 Because of the anatomical relationship of the optic nerve’s dural and arachnoid sheaths, decreased visual acuity may occur with inflammation around this area. One study found that, initially, poor vision was the most important risk factor for a negative visual outcome.24 Other symptoms include photophobia and increased lacrimation.9
Scleritis is a more painful condition than episcleritis, and the pain may appear disproportionate to clinical findings.12 Patients describe it as a deep, boring pain that may radiate to the face, cheek and jaw.2,9,12,16 Often, it is worse at night and is exacerbated with eye movement.2,9,21 Investigators discovered a significant increase in pain severity with HZV scleritis cases vs. those with idiopathic etiologies.22
Though more agonizing, scleritis may ironically reduce corneal sensation.26 Researchers cite a decrease in conjunctival sensation in areas of previous inflammation, with the exception of HZV scleritis, which blunted sensation in both active and inactive states. This occurred due to the cornea and conjunctiva sharing the same classes of sensory receptors and the increased likelihood of corneal involvement with HZV.26
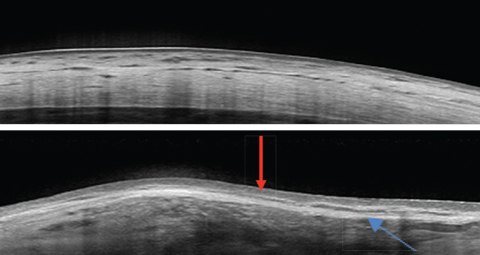 |
| These images show a normal sclera, above, vs. nodular episcleritis, below. Note the increased thickness of the episclera (red arrow) while the scleral thickness is unaffected. The elongated hyporeflective area (blue arrow) could indicate edema in the presence of a thickened sclera. In this case, it was due to a scleral plaque. Click image to enlarge. |
Clinical Presentation
Each of these conditions have slightly different presentations:
Episcleritis. This is usually diffuse or simple with benign, mild inflammation that resolves within days to weeks.2,4 Nodular episcleritis, frequently located between the palpebral fissures, is more painful and lasts longer.1,4 Epiphora may also be present. Because epislceritis involves the conjunctival and superficial episcleral plexi, the affected area appears bright red, unlike the characteristic bluish-violet hue associated with the deep episcleral plexus involvement in scleritis.9,21 Additionally, intraocular pressure (IOP) may be elevated due to increased episcleral venous pressure (EVP).27
Scleritis. This varies depending on the location and nature of involvement. Uveitis occurs in up to 40% of all scleritis patients and is usually seen with the necrotizing type.9 Keratitis is present in 14% to 37% of scleritis patients and usually targets the adjacent peripheral cornea and may include thinning, infiltrates and interstitial keratitis.3,21 Increased IOP from elevated EVP or trabeculitis leads to glaucoma in 9% to 13% of scleritis patients.2,9,21 Less than 10% progress from one type of scleritis to another.3
Vascular congestion and a tender globe follow the insidious onset of diffuse anterior scleritis, the most common and the least severe form.1,3,9,21 A firm, immobile focal area of inflammation denotes nodular anterior scleritis. As with nodular episcleritis, it usually presents within the palpebral fissure and can be single or multiple.1,9,21
Necrotizing anterior scleritis marks the most severe form and the greatest threat to the integrity of the eye.9 Intense vasculitis with closure of the deep episcleral vascular plexus leads to necrosis, tenderness and extreme pain.1,2,9 The translucently thin sclera highlights the choroid’s bluish hue. Necrotizing anterior scleritis is most likely associated with systemic disease and peripheral ulcerative keratitis.1,3,16,21
Although now considered rare due to improved therapies for RA, scleromalacia perforans lacks surrounding inflammation and stems from obliterative arteritis involving the deep episcleral plexus.9,16 Necrotic scleral plaques may appear near the limbus, and severe thinning yields a brown color, along with a high amount of astigmatism.1,2,9
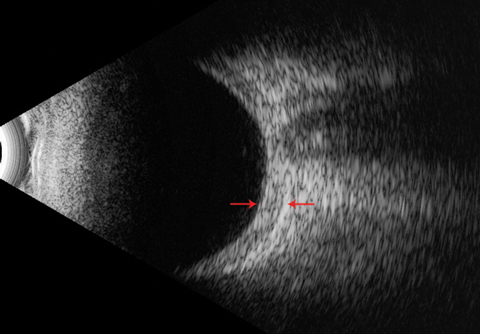 |
| This B-scan ultrasound shows a thickened posterior sclera (arrows) with an absent ‘T’ sign. |
Diagnostic Tools
In addition to the clinical exam, clinicians can use optical coherence tomography (OCT), ultrasound biomicroscopy and B-scan ultrasound to help differentiate between episcleritis and scleritis. OCT, for example, can provide objective cross-sectional images and help to describe characteristic findings with anterior scleritis.19 Using spectral-domain (SD) OCT, researchers note consistent findings of hyporeflective spaces secondary to edema and blood vessels, variations in reflectivity of tissues and dilated deep episcleral vessels.19 OCT further shows treatment results in normalization of scleral tissue.19
Other researchers noted superficial layer involvement in episcleritis, but dilated vessels in the deeper vascular network with scleritis.7 Another study found the scleral thickness in episcleritis and scleritis averages 825µm and 882µm, respectively (the normal sclera is roughly 750µm with a standard deviation of nearly 70µm).5 Clinicians should be cautious about basing a diagnosis solely on thickness, however, as nodular cases will skew the measurements. The key is looking for pockets of edema in the sclera.5
High-frequency ultrasound biomicroscopy can be a useful tool in distinguishing between episcleritis and scleritis. Tissue penetration is better with this modality, but it comes at the expense of decreased resolution compared with OCT.5 Early adopters found that episcleral thickening could be differentiated from scleral involvement.28
Unlike with other scleritis forms, the observable eye in posterior disease may be nonerythematous.18 Other dissimilarities include its twice-as-often unilateral presentation and pain that does not correlate with the severity of inflammation.1,2,29,30 Because it is not visible, clinicians must depend on other procedures when assessing posterior scleritis. A dilated fundus exam may reveal choroidal folds, serous retinal detachments, choroidal detachments, macular or optic disc edema and vitreous cells.3,21,29 B-scan ultrasound demonstrates posterior thickening with fluid collecting in the sub-Tenon’s space, producing the pathognomonic ‘T-sign.’31 Roughly 40% of posterior scleritis patients exhibited this finding and more than half had posterior scleral wall thickness greater than 2mm.29
 |
| This 58-year-old male with diffuse anterior scleritis was placed on naproxen 500mg BID, but continued symptoms warranted an increase to TID. The inflammation persisted, and we chose to change from naproxen to indomethacin 50mg TID. The patient responded well and the condition resolved. |
Treatment
Once the proper diagnosis is clear, clinicians can initiate the proper treatment for the patient:
Episcleritis. Because episcleritis is usually a self-limiting condition with a nearly 20% resolution rate without treatment, patient education or topical lubricants may suffice.1,2 Research suggests topical non-steroidal anti-inflammatory drugs (NSAIDs) provide no benefit over artificial tears.32 Although this condition can be self-limiting, many clinicians initially treat with a topical steroid. If the inflammation continues, the dosing frequency may be increased or the patient can be switched to a more potent topical steroid.1,2,33 Only with the failure of these regimens should practitioners resort to oral NSAID therapy, which occurs mainly in patients with a known systemic association.1,33
Scleritis. Before initiating any treatment, clinicians should first determine if the scleritis is infectious, as drugs such as oral steroids may worsen the condition. A thorough history into any ocular surgery or trauma should be obtained as well. Clinically, a mucopurulent discharge or scleral abscess may be sufficient signs to signal infection in the absence of a tissue culture and sensitivity test.23 A complete blood count may reveal elevated white blood cells. HZV, as the most common infectious cause, responds well to oral acyclovir or famciclovir and should resolve the symptoms within several weeks or less.22
Unlike with episcleritis, NSAIDs may be a first-line treatment for diffuse or nodular, idiopathic and non-necrotizing anterior scleritis.3,4,9 If the patient sees no improvement, clinicians should prescribe a different NSAID before advancing to the next therapeutic level.2 The selective COX-2 inhibitors such as celecoxib are good options when adverse gastrointestinal side effects are a concern. While some research suggests steroids provide no benefits, a study of a series of non-necrotizing anterior scleritis cases showed topical steroids resolved nearly half of the cases, but often they require additional treatment.2-4,34 Durezol (difluprednate, Alcon/Novartis), the topical ophthalmic steroid emulsion that exhibits enhanced penetration and bioavailability, has not been studied for scleritis use.
Oral corticosteroids are the next therapeutic step for refractory cases, those with necrotizing signs or posterior scleritis.9,33 Typical oral dosages range from 1mg/kg/day to 1.5mg/kg/day until the inflammation is controlled.1,4 Given the nocturnal pain associated with scleritis, it may be wise to spread the dose over the day vs. a morning-only delivery.1 Tapering depends on dosage, duration of use and practitioner discretion. Most practitioners use the lowest possible dose for the shortest possible time, as long-term steroid treatment should be avoided due to the deleterious side effects.
Other routes of steroid introduction include intravenous (IV) and periocular injection. Necrotizing scleritis, for example, may warrant IV methylprednisolone.3,4 Though not routinely employed, periocular injection of steroids can be effective in controlling inflammation in non-necrotizing anterior scleritis without the systemic side effects.3
Those with an associated systemic condition or the necrotizing type typically need immunosuppressive therapy or biological agents.35 Examples include methotrexate, azathioprine, cyclophosphamide, mycophenolate mofetil, infliximab and rituximab.
There are several concerns to remember regarding treatment:
Recurrence is not uncommon. For example, research shows younger patients tend to have recurrence sooner and more frequently.29 Diffuse anterior scleritis is least likely to recur, followed by the nodular version of the same process.8 Drug tapering may initiate recurrence.
Immunosuppressive drugs increase the risk for a secondary infection. What starts out as an idiopathic condition could turn infectious, which should be considered in patients who respond well initially but then worsen.
Malignancy may mimic treatment-resistant scleritis.2,18 This could include intraocular tumors such as melanomas or, rarely, conjunctival tumors and lymphoma.9
The chronic use of NSAIDs is not benign. Clinicians should regularly monitor liver and kidney function, as well as blood pressure in hypertensives.2 Gastric irritation may require additional medications.
Nearly 50% of patients with scleritis, and more than 30% of those with episcleritis, have an underlying systemic disease.2,4,9,21,33 Therefore, treatment must include a suitable workup with the understanding that scleritis is the first manifestation of systemic disease in only 15% of patients (Table 1).3 Many clinicians defer this testing in episcleritis cases unless it is refractory or has a high rate of recurrence. Once discovering systemic disease, clinicians should consult the proper specialist for management. Some state RA is the most common systemic condition associated with scleritis, while others found RA with vasculitis—particularly granulomatosis with polyangiitis (GPA)—was the number one cause.12,33 Still others recorded GPA and relapsing polychondritis as top offenders.3
A thorough case history, a review of patient symptomatology, comparative clinical findings and further diagnostic tools are keys to helping clinicians successfully differentiate between episcleritis and scleritis. The right diagnosis and prompt treatment leads to improved outcomes, as does adhering to a multidisciplinary approach when applicable.
Dr. Williamson is the residency supervisor at the Memphis VA Medical Center and is adjunct faculty at multiple optometry schools.
1. Bowling B. Kanski’s Clinical Ophthalmology. Philadelphia: Saunders; 2015. |

