FDA to Restructure Device Regulations
Changes are in the air to ensure the right policies are in place to encourage safe and effective innovation.
By Bill Kekevian, Senior Editor
The Food and Drug Administration (FDA) has announced it will begin publishing new guidelines on how it regulates health care technologies, such as mobile apps, fitness trackers and clinical decision support software, as part of what it’s calling its “digital health innovation plan.” The guidelines may replace premarket regulation of certain devices, according to the agency.
In a blog post, FDA Commissioner Scott Gottlieb, MD, wrote that the “FDA should […] promote the public health through policies that are clear enough for developers to apply them on their own, without having to seek out, on a case-by-case basis, FDA’s position on every individual technological change or iterative software development.”
“The new guidance will facilitate development of smartphone apps, wearable devices and data-driven diagnosis across health care, including optometry,” says Brian Chou, OD, of San Diego, Calif. “ODs should pay attention to this, since it’ll likely have implications with online refraction and telemedicine.”
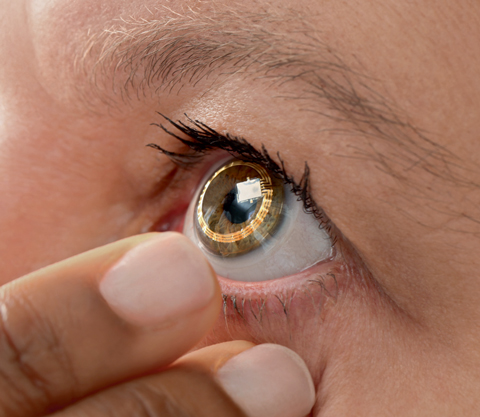 |
| Sensimed’s Triggerfish smart contact lens, designed to detect tiny fluctuations in the eye’s volume, is just one such device that may be influenced by the FDA’s proposed new guidelines on health care technology. Click image to enlarge. Photo: Sensimed |
According to Dr. Gottlieb’s post, the FDA is considering employing a third-party certification program so that products categorized as “low-risk” can be marketed without FDA premarket review and products designated as “higher-risk” can be marketed with a “streamlined” FDA premarket review.
A representative from the agency confirmed that “the program is currently in development,” but couldn’t share any details about the definition of low- and higher-risk devices. Dr. Gottlieb’s blog post hints that “clinical administrative support software and mobile apps that are intended only for maintaining or encouraging a healthy lifestyle” fall outside the scope of FDA regulation.
According to an estimate cited on the blog post, 165,000 health-related apps were made available for Apple or Android smartphones in 2016. Added to that, device manufacturers have a wide variety of eye care related medical devices in the works that may soon impact optometric practice. These devices range from retinal implants and drainage stents to smart contact lenses that measure glucose levels or report on a patient’s glaucoma, and online refraction testing software.
“Since the FDA is signaling that it’ll take a hands-off approach toward ‘low risk’ digital health devices, the question is whether the FDA considers refraction and disposable contact lens Rx renewal under that category,” Dr. Chou says. “If so, expect to see even more digital health devices for online refraction and contact lens Rx renewal.”
| Gottlieb S. Fostering medical innovation: a plan for digital health devices. FDA Voice. https://blogs.fda.gov/fdavoice/index.php/2017/06/fostering-medical-innovation-a-plan-for-digital-health-devices. June 15, 2017. Accessed June 28, 2017. |
Substance P: A New Test for Neuropathy
New research suggests low levels of substance P—a nerve cell signaling molecule found in tears—may indicate an increased risk of diabetic corneal neuropathy. After measuring substance P levels in the tears of nine diabetes patients and 17 controls, investigators found the patients with diabetes had lower levels of substance P compared with the controls.
“Substance P plays a role in the maintenance and nutrition of the cornea by promoting migration and proliferation of corneal epithelial cells,” says Maria Markoulli, PhD, MOptom, study author and senior lecturer at the School of Optometry and Vision Science at the University of New South Wales, Sydney, Australia. “The reduction in substance P levels in diabetes may contribute to the poorer wound healing and increased susceptibility to corneal neurotrophic ulcers that is seen in diabetes.”
Challenging Today’s Tools
Confocal microscopy further revealed participants with diabetes had moderately lower corneal nerve fiber density than the controls.
“The association with corneal nerve density and substance P in the tear film led us to hypothesize that substance P could be a marker of corneal neuropathy,” says Dr. Markoulli. “Being able to detect those with corneal neuropathy may help us implement systemic management sooner.”
Although confocal microscopy is the go-to instrument to assess corneal neuropathy, it’s usually only available in the research setting, according to Dr. Markoulli. A more practical tool, such as substance P testing, could be far more applicable in the clinic, she says.
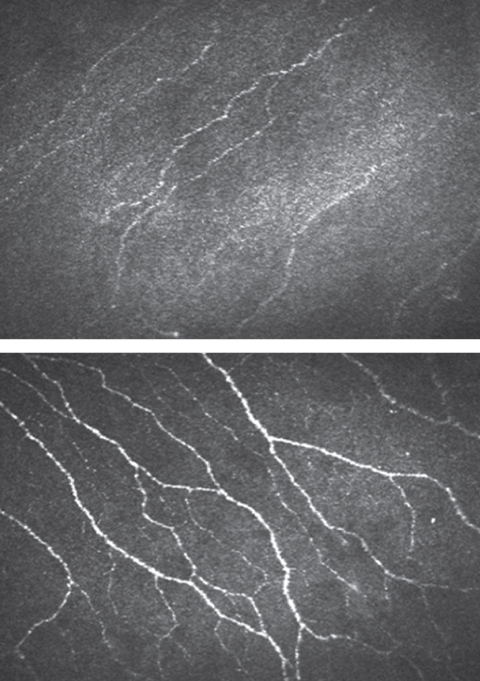 |
| Confocal microscopy shows lower nerve fiber density in patients with diabetes (top) compared with controls (bottom). Click images to enlarge. Images: Maria Markoulli, PhD, MOptom |
Tomorrow’s Treatment
“Further research of this concept may ultimately result in the development of a topical medication composed of substance P,” says Richard Zimbalist, OD, of Columbia, Mo. “While such a medication may obviously be beneficial for diabetic neurotrophic ulcers, it is feasible to consider the potential implications for corneal surgeries, ulcers and other conditions that rely upon epithelial migration and wound healing.”
While substance P testing could one day be a useful noninvasive test to help assess for peripheral neuropathy in patients with diabetes, the research is still in its nascent stages, says Dr. Markoulli. “We are now running a larger study and exploring other neuropeptides, as well as the link with peripheral neuropathy. At this stage, however, this is all speculation and a lot more work needs to be done to confirm this.”
| Markoulli M, You J, Kim J, et al. Corneal nerve morphology and tear film substance P in diabetes. Optom Vis Sci. 2017;94(7):726-31. |
Treating Conjunctivitis: Ease Up on Antibiotics
Nearly 60% of patients are incorrectly prescribed an antibiotic to treat acute conjunctivitis, according to a new study. After looking at 340,372 patient records with a diagnosis of acute conjunctivitis, researchers found 58% were prescribed antibiotics, approximately 20% of whom received a combination antibiotic-corticosteroid drop. Not only are antibiotics rarely necessary, according to the study authors, antibiotic-corticosteroids are contraindicated for acute conjunctivitis.
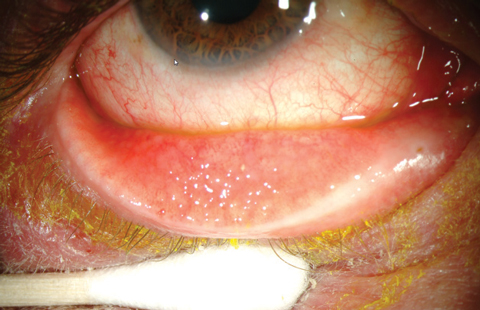 |
| Antibiotics are not a proper treatment for most cases of viral conjunctivitis; yet, new data suggests PCPs still prescribe antibiotic eye drops. Photo: Marc Bloomenstein, OD |
The study notes most patients (83%) were diagnosed by a primary care provider (PCP) such as a family physician, pediatrician or urgent care provider, not an ophthalmologist or optometrist. The researchers speculate the challenge of differentiating bacterial conjunctivitis from the viral and allergic forms may be one reason PCPs overprescribe antibiotics. Even mild cases of bacterial conjunctivitis don’t warrant antibiotics, the researchers said, as they usually resolve on their own within two weeks without treatment.
The findings suggest “a pressing need to educate all health care providers better about the fact that most patients with of acute conjunctivitis do well with conservative management and do not require immediate antibiotic therapy,” the study says.
In addition to practitioner prescribing habits, the investigators took a closer look at patient prescription filling habits. Sixty-eight percent of patients diagnosed by an urgent care physician filled their prescription, compared with 56% of those diagnosed by a family physician, 44% by an optometrist and only 36% by an ophthalmologist.
While the researchers do not speculate on why such a disparity exists for prescription fulfillment based on the diagnosing practitioner, the study as a whole highlights the need for more education for both practitioners and patients alike on the proper management of acute conjunctivitis.
The authors also note several limitations to the data. For one, the records provide no data on the details that led to the prescription. “No doubt some of the enrollees who had been diagnosed with acute conjunctivitis would have been appropriate candidates for antibiotic therapy,” according to the study. In addition, the results only included patients with commercial health insurance, did not include data on free medication samples frequently dispensed in clinics and excluded inpatient and hospital-acquired cases of acute conjunctivitis.
| Shekhawat NS, Shtein RM, Blachley TS, Stein JD. Antibiotic prescription fills for acute conjunctivitis among enrollees in a large United States managed care network. Ophthalmology. 2017;124:1099-107. |
Drops for Dry Eye: Think Cord Blood SerumA new study suggests cord blood serum (CBS) eye drops could improve corneal nerve morphology and subjective symptoms in patients with severe ocular surface disease (OSD). After 20 OSD patients used CBS drops for two months, researchers found their Ocular Surface Disease Index, visual analog scale and Oxford grading values significantly decreased, while corneal sensitivity, Schirmer’s test score and tear break-up time significantly increased. In vivo confocal microscopy (IVCM) further revealed improved corneal nerve morphology, including a higher number of total nerves, lower nerve tortuosity and fewer giant epithelial cells, neuromas and beading post-treatment. Finally, patients with neuromas and higher levels of dendritic cells before treatment experienced a greater OSDI reduction after treatment. Although limited by a small sample size and lack of controls, the study suggests CBS eye drops may be a promising treatment option. In addition, the researchers note the IVCM data provided subclinical metrics for evaluating ocular surface epithelial and neural alterations and may “help to diagnose the severity stage of DED, select patients appropriately, and monitor the course of treatment.” Giannaccare G, Buzzi M, Fresina M, et al. Efficacy of 2-month treatment with cord blood serum eye drops in ocular surface disease: an in vivo confocal microscopy study. Cornea. 2017;36(8):915-21. |
OSA Patients at Risk for DR Progression
Obstructive sleep apnea (OSA) patients also diagnosed with Type 2 diabetes are at increased risk for progression to pre-proliferative diabetic retinopathy (DR) and are more likely to progress to sight-threatening DR within four years, according to a new report.1,2
In the prospective study, researchers evaluated 230 patients with Type 2 diabetes for retinopathy using retinal imaging, taken during routine care.1,2 To evaluate OSA, patients were given a home-based, multi-channel cardiorespiratory portable device to use a single time, and the results were scored using American Academy of Sleep Medicine guidelines to discern the presence of the condition.1,2 The study excluded subjects suffering from any respiratory condition or who had a previous diagnosis of OSA.1,2
 |
| Patients with OSA and Type 2 diabetes are at an increased risk for developing sight-threatening DR. Photo: Mohammad Rafieetary, OD |
Results
According to the study, sight-threatening DR was more common in patients with both Type 2 diabetes and OSA compared with those diagnosed with Type 2 diabetes alone.1,2 They observed DR in 42.9% of patients with both OSA and Type 2 diabetes, compared with only 24.1% of patients in the diabetes-only category.1,2
The research also reveals that patients with OSA and Type 2 diabetes, compared with those with diabetes only, are at increased risk of sight-threatening DR over a period of three years and seven months.1,2 At follow-up, roughly 43 months later, the patients with OSA were 18.4% more likely to develop moderate to severe DR compared with those without OSA at just 6.1%.1
Finally, the study shows patients who received continuous positive airway pressure treatment had a lower risk of developing advanced DR compared with patients who did not receive the therapy.1,2
Clinical Significance
The study provides crucial observations, says Mohammad Rafieetary, OD, of Charles Retina Institute in Germantown, Tenn. “We already know that OSA is a major risk factor for ischemic optic neuritis, a visually debilitating disease, and that this disease is, along with diabetic retinopathy, another potential complication of diabetes.”
Hypoxia or ischemia is also a major factor for progression and poor prognosis, he explains. “It is sensible to assume any factors that cause oxygen denervation can result in worsening of retinopathy and increased vision loss,” he says. “These also include smoking and increased BMI and obesity.”
Practitioners must pay attention and address all of these potential complicating factors for better patient management, Dr. Rafieetary suggests.
1. Altaf QA, Dodson P, Ali A, et al. Obstructive sleep apnoea and retinopathy in patients with Type 2 diabetes: a longitudinal study. Amer J Resp Crit Care Med. 2017 June. [Epub ahead of print]. |
In the newsNew research suggests young adult patients with myopia with specific binocular vision disorders may benefit from orthokeratology (OK) more so than single vision soft contact lenses (SCLs). Investigators compared 17 OK wearers with 17 single vision SCL wearers and found those wearing OK lenses were significantly more exophoric, had better accommodation accuracy and lower accommodative lags at near, compared with the single vision SCL wearers. Gifford K, Gifford P, Hendicott PL, Schmid KL. Near binocular visual function in young adult orthokeratology versus soft contact lens wearers. Cont Lens Anterior Eye. 2017;40(3):184-9. A new study suggests pattern visual-evoked potentials (VEP) could help clinicians identify patients with convergence insufficiency and a history of concussion. Researchers used pattern VEP to test 79 patients based on a diagnosis of convergence insufficiency/binocular dysfunction—35 of whom reported a history of concussions. They found two different pattern VEP models discriminated between concussed and nonconcussed groups. With further research, VEP could become a valuable diagnostic tool for these patients. Poltavski D, Lederer P, Cox LK. Visually evoked potential markers of concussion history in patients with convergence insufficiency. Optom Vis Sci. 2017;94(7):742-50. When studying 300 families with retinitis pigmentosa (RP), researchers found more than 34% of the Hispanic patients exhibited a specific arestin-1 gene mutation—and all of them hail from Southwestern United States. The researchers hope further studies into the gene mutations responsible for RP could lead to individualized gene therapy. Sullivan LS, Bowne SJ, Koboldt DC, et al. A novel dominant mutation in SAG, the arrestin-1 gene, is a common cause of retinitis pigmentosa in Hispanic families in the Southwestern United States. Invest Opthalmol Vis Sci. 2017;58(5):2774. |

