23rd Annual Glaucoma ReportFollow the links below to read the other articles from our 23rd annual Glaucoma Report:Glaucoma Surgery: Are You Ready to Refer? 10-2 Visual Field Testing: A Tool for All Glaucoma Stages Mastering MIGS: Today and Tomorrow |
As far as we know, intraocular pressure (IOP) is the only modifiable risk factor with respect to altering glaucoma’s characteristic death of retinal ganglion cells.1-15 IOP quantifies the balance between aqueous humor (AH) secretion by the ciliary body epithelia and its drainage through the conventional, pressure-sensitive pathway and unconventional, pressure-insensitive or “uveoscleral” pathways.6,13-17 IOP will increase along with any resistance to aqueous outflow.18 This can result from age-related cellular dysfunction in the conventional outflow pathway or a normal outflow system unable to handle overproduction.18 The rate of aqueous production is dependent on blood flow to the ciliary body and the rate of active secretion from the ciliary epithelium.12 The estimated turnover rate is 1.0% to 1.5% (2.5ml/minute) of the anterior chamber volume per minute.10,13 Research shows a 20% to 30% decrease in IOP can significantly reduce the risk of progression of primary open-angle glaucoma (POAG).1
Since altering other mechanisms in the pathophysiology of the disease is not possible without profound risk to the patient, lowing IOP is the only means of arresting disease progression.13-18 In the United States, the current guidelines for the initial intervention of newly discovered cases of POAG recommend a first-line approach with topical ocular hypotensives.27 To that end, three new agents that are under investigation aim to decrease IOP without upsetting the physiology. If successful, these medications could help keep the primary outflow pathway functioning.
This article introduces these new medical interventions and explains how they can impact IOP for glaucoma patients.
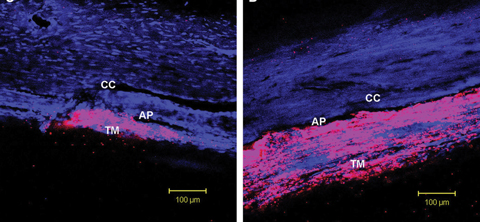 |
| Rho-kinase inhibitors appear to lower IOP by inducing cellular relaxation and disrupting focal adhesions in the TM and the endothelial lining of Schlemm’s canal. Click image to enlarge. Photo: Patrick A. Scott, OD, PhD |
Aqueous Outflow
The conventional or trabecular meshwork (TM) outflow pathway accounts for 80% to 90% of aqueous outflow under normal physiological conditions.1-17 The uveoscleral outflow pathway covers the remaining 10% to 20%.7 Anatomically, the TM can be separated into distinct regions based on location and function. The uveal and corneoscleral meshwork consist of arrays of lamellae comprised of fenestrated collagen beams
covered by endothelial-like cells, with loose extracellular matrix (ECM) occupying the spaces between the cells of the adjacent beams.19-22 The ECM provides a channel for AH to cross the juxtacanalicular tissue (JCT) and exit the anterior chamber through Schlemm’s canal (SC), where the AH is eventually drained into the venous circulation.7-17 The ECM is an active structure, possessing many bioactive molecules that influence outflow resistance. This activity in the extracellular environment is linked to alterations in the intracellular actin cytoskeleton and vice-versa.7-17 For normal patients, resistance to aqueous flow is greatest in the JCT region, the inner wall of SC or both.7-17
Regulation of aqueous outflow in the primary outflow pathway is mitigated by the interaction of two cell types in the JCT: the TM and SC endothelia. TM cells express smooth muscle-like properties, including contractility, electro-mechanical characteristics and expression of actin and myosin specific to smooth muscle tissue.1 This highly structured cellular actomyosin system affects the overall contractile tone of the tissue influencing outflow resistance.12 Research confirms that actin depolymerization coupled with decreased cell-ECM interactions and myosin II phosphorylation within cells of the trabecular pathway increases AH outflow, consequently decreasing IOP.8,12 The majority of aqueous flowing across the SC endothelia is thought to pass through micron-sized pores. SC cells are highly contractile, and increased contraction greatly increases their cell stiffness. Altered cell stiffness modifies pore formation, ultimately affecting downstream egress of AH from the eye.7-17
Outflow Mechanics
While normal individuals will see a reduction of aqueous outflow facility through the TM of approximately 7% to 10% per decade, POAG patients develop an increased resistance to outflow.7-17 Research supports a link between cytoskeletal integrity within the cells of the trabecular pathway and AH outflow through that route.8 Histological changes observed in POAG patients, possibly contributing to decreased aqueous outflow, include declining number of TM cells, increased and changed ECM components, deposition of extracellular plaques and stiffening of the TM with decreased contractility force of the elastic fibers.7-17
Specifically, as the smooth muscle-like properties of TM cells facilitate dynamic tissue restructuring, marked loss of these cells, which seems to be exaggerated in glaucoma, leads to fusion and thickening of the trabecular lamellae, impairing its function.1-19 The deposition of extracellular plaques within glaucomatous JCT ECM is similar to the characteristics of the process of fibrosis. These aberrant accumulations adhere to the sheaths of the elastic fibers and their connections to the inner endothelium wall of SC.13-16 Research shows these increased cell-cell junction adherences between SC cells and accumulated ECM underlie the pathological increased resistance to aqueous outflow.13-16 In addition, the subcortical SC cell stiffness is elevated by as much as 50% in glaucomatous eyes.13-16 This correlates with decreased pore density, impairing the egress of AH from the eye.7-16
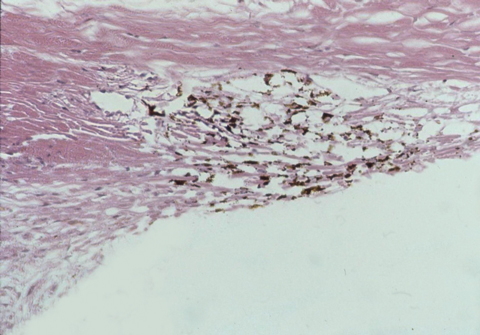 |
| Rho-kinase inhibitors improve drainage through the trabecular meshwork, seen here under a microscope. Click image to enlarge. Photo: Thomas Freddo, OD, PhD |
Current Approaches
Topical and oral glaucoma medications lower IOP by reducing production, increasing AH outflow, or both. These include topical prostaglandin analogs (PGAs), beta-blockers, topical and oral carbonic anhydrase inhibitors, topical sympathomimetics and topical miotics.
PGAs are the most efficacious at lowering IOP, and work primarily by increasing uveoscleral outflow. Although some studies show they may alter resistance in TM outflow, that effect is minimal.29-32 In the United States, miotics are the only available medications that have a mechanical effect on the conventional outflow pathway. These agents work as parasympathomimetics, contracting both the iris constrictor and ciliary muscles, which increases the mechanical pull on the TM and opens the meshwork’s parasellar spaces.33-36 While the medicines in this class are inexpensive, unfortunately they require frequent dosing and carry significant ocular side effects such as brow ache, small pupil size (which can accentuate the effects of cataract in older patients) and increased risk of retinal detachment.34,35 New therapeutic approaches aim to harness this pathway with less deleterious side effects.33-41
Recent investigations suggest the TM outflow tissues are avascular and dependent on AH to supply antioxidants, growth factors, chemokines and nutrients.9,42 Ironically, therapies that suppress aqueous production or enhance uveal and scleral outflow decrease the supply of AH across the outflow tissues.9,42 Reduction of IOP unquestionably protects the optic nerve; however, researchers wonder if nutrient deprivation from fluid reduction, in the long run, creates a greater than normal degradation of the trabecular outflow pathway and in turn greater risks over time.9,42
While surgical options are available for cases that exhibit progression in the setting of maximum topical therapy, new agents can decrease IOP without upsetting the physiology.
ROCK Inhibitors
Rhopressa (netarsudil ophthalmic topical solution 0.02%, Aerie Pharmaceuticals), currently in Phase III clinical trials, is unlike the current first-line therapies that focus on enhancing unconventional aqueous outflow through the uveoscleral pathway. Data from preclinical and clinical trials suggests that Rhopressa, dosed once daily, lowers IOP via four mechanisms.38 First, it is a rho kinase (ROCK) inhibitor. ROCK inhibitors increase actomyosin contraction in smooth muscle-like cells, including the myofibroblast-like cells of the TM, increasing outflow.39 Second, preclinical trials showed netarsudil had antifibrotic effects on TM cells, producing increased perfusion through the TM.37 Third, netarsudil also lowered episcleral venous pressure in animal studies, in turn lowering IOP by reducing resistance to AH outflow.37 The fourth mechanism of IOP reduction is due to norepinephrine transporter (NET) inhibition.38 NET inhibition occurs in the ciliary body, resulting in increased norepinephrine levels which decrease AH secretion via activation of α2 adrenergic receptors.43
The mean IOP-lowering effect of netarsudil during Phase II and Phase III clinical trials was 5.5mm Hg.40 In the Rocket 1 clinical study, netarsudil was found to be inferior to timolol at higher levels of baseline IOP.38 The drug seems to possess better IOP-lowering capabilities at IOPs less than 25mm Hg. This may result in FDA labeling for use in cases where IOP lowering is necessary but the untreated measurement is less than 25mm Hg.
Netarsudil is currently under analysis in two Phase III clinical trials (Rocket 3 and Rocket 4). Rocket 3 is a 12-month safety-only study in Canada. Rocket 4 is designed to provide adequate six-month safety data for regulatory filing purposes in Europe.38 In addition, the Rocket 4 top-line 90-day efficacy data successfully demonstrated non-inferiority to timolol at its primary endpoint range.38
Throughout the clinical trials, there were no drug-related serious adverse events and no evidence of treatment-related systemic effects. The most common side effect in the treatment group was conjunctival hyperemia (seen in approximately 48% of the cohort).38 Other side effects, which were noted in 3% to 5% of the treatment group, included cornea verticillata, conjunctival hemorrhage, increased lacrimation, erythema of eyelid and blurred vision.38 Since side effects such as these are possible, patient education is of the utmost importance. While these are undesirable, their possibility alone should not prevent clinicians from suggesting Rhopressa for treatment.
Another ROCK Option
Preclinical and clinical research demonstrates that Roclatan (netarsudil/latanoprost ophthalmic solution 0.02%/0.005%, Aerie Pharmaceuticals) works on all known mechanisms of IOP reduction; decreasing aqueous production, increasing outflow from the TM, increasing outflow from the uveal scleral outflow pathway and reducing episcleral venous pressure.38
Roclatan has undergone multiple Phase III clinical trials: Mercury 1, Mercury 2 and Mercury 3. Mercury 1 is a 12-month safety trial in 718 patients with a 90-day efficacy readout. During this trial, Roclatan showed superiority to each of its components, achieving up to a 3mm Hg greater IOP lowering effect.38 Mercury 2 is a 90-day efficacy trial that commenced in March 2016.38 Mercury 3 is a registration trial not needed for FDA approval or commercialization, but designed to help with approval and release in Europe.38
Reducing Resistance
Vyzulta (latanoprostene bunod ophthalmic solution 0.024%, Bausch + Lomb), works as a nitric oxide (NO) donating prostaglandin F2α analog.44,45 Upon instillation, latanoprostene bunod is hydrolyzed by endogenous corneal esterases into latanoprost acid and butanediol mononitrate, which is further metabolized to NO and the inactive 1,4-butanediol.45,46 NO reduces IOP by enhancing aqueous outflow through the TM and SC. Latanoprost acid, the active metabolite in latanoprost, increases the presence of matrix metalloproteinases (MMPs). MMPs degrade collagen, specifically types I, III and IV. This degradation reduces outflow resistance through the uveoscleral pathway, ultimately lowering IOP.46
The most notable clinical trial for latanoprostene bunod was Voyager.42 This Phase II clinical trial demonstrated latanoprostene’s superiority over latanoprost.44,45 Constellation, a Phase II, small-scale trial demonstrated superiority over timolol twice daily.45 Phase III trials include Apollo, Lunar and Jupiter.
Apollo, a larger trial, compared latanoprostene bunod with timolol and demonstrated superiority in overall IOP reduction. The study had a greater number of participants with IOP less than or equal to 18mm Hg and showed a higher percentage of IOP reduction greater than or equal to 25%.44 These clinical trials show Vyzulta has the potential to provide an additional 1mm Hg to 3mm Hg drop in IOP, compared with the addition of latanoprost and timolol.45 Twenty-two percent of subjects reported at least one adverse ocular event. These events were mild, transient and consistent with those seen caused by other topical glaucoma medications, including conjunctival hyperemia, eye irritation and eye pain.44,45
The incidence of glaucoma continues to rise and the more ammunition provided to the armamentarium the better. Surgical interventions, such as cataract extraction, filtering procedures and the placement of drainage devices, increase risk to the patient. These new topical therapies, both as primary choices or as an addition to an established course of therapy have shown great promise for reducing IOP in the setting of once-daily dosing with excellent safety profile.
Their ability to act on the conventional and unconventional outflow mechanisms in addition to having other IOP-lowering mechanisms not available with current topical treatment modalities makes them valuable and anticipated.
Dr. Rebar is coordinator of residency programs in Primary Care and Cornea & Contact Lens at Salus University
Dr. Gurwood is a clinical director and professor with the Eye Institute at Salus University.
| 1. Quigley H. New paradigms in the mechanisms and management of glaucoma. Eye. 2005;19(12):1241-8. 2. Quigley H, Broman A. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-7. 3. Tham Y, Li X, Wong T, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90. 4. Kapetanakis V, Chan M, Foster P, et al. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol. 2016;100(1):86-93. 5. Weinreb R, Aung T, Medeiros FA. The pathophysiology and treatment of Glaucoma: A Review. JAMA. 2014;311(18):1901-11. 6. Janssen SF, Gorgels TG, Ramdas WD, et al. The vast complexity of primary open angle glaucoma: Disease genes, risks, molecular mechanisms and pathobiology. Prog Retin Eye Res. 2013 Nov;37:31-67. 7. Alm A, Nilsson SF. Uveoscleral outflow - a review. Experimental Eye Research. 2009;88(4):760-768. 8. Brubaker RF. Targeting outflow facility in glaucoma management. Surv Ophthalmol. 2003;48(Suppl 1):S17-20. 9. Stamer WD, Braakman ST, Zhou EH, et al. Biomechanics of Schlemm’s canal endothelium and intraocular pressure reduction. Prog Retin Eye Research. 2015;44(1):86-98. 10. Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010 Sep;4:52–9. 11. Kanski J. Clinical ophthalmology: a systematic approach, 6th ed. Edinburgh: Elsevier Butterworth- Heinemann; 2007. 12. McDougal DH, Gamli PD. Autonomic control of the eye. Compr Physiol. 2015;5(1):439–73. 13. Gabelt BT, Kaufman PL. Aqueous humor hydrodynamics. In: Hart WM, Ed. Adler’s Physiology of the Eye, 9th ed. St. Louis, MO: Mosby; 2003. 14. Johnson M. What controls aqueous humor outflow resistance? Exp Eye Res. 2006;82(4):545-57. 15. Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88(4):648-55. 16. Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate. Prog Retin Eye Res. 1999;18(1):91-119. 17. Tian B, Gabelt BT, Geiger B, Kaufman P. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res. 2009;88(4):713-17. 18. Keller KE, Acott TS. The juxtacanalicular region of ocular trabecular meshwork: A tissue with a unique extracellular matrix and specialized function. J Ocul Biol. 2013;1(1):3-15. 19. Keller KE, Aga M, Bradley JM, et al. Extracellular matrix turnover and outflow resistance. Exp EyeRes. 2009; 88(4):676-682. 20. Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88(4):769-75. 21. Roy Chowdhury U, Hann CR, Stamer WD, Fautsch MP. Aqueous humor outflow: dynamics and disease. Invest Ophthalmol Vis Sci. 2015;56(5):2993-3003. 22. Overby DR, Zhou EH, Vargas-Pinto R, et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc Natl Acad Sci USA. 2014;111(38):13876-81. 23. Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am J Physiol Cell Physiol. 2008;295(5):C1057-70. 24. Brubaker RF. Introduction: three targets for glaucoma management. Surv Ophthalmol. 2003;48(Suppl 1):S1-2. 25. Kameda T, Inoue T, Inatani M et al. The effect of rho associated protein kinase inhibitor on monkey Schlemm’s canal endothelial cell. Invest Ophthalmol Vis Sci. 2012;53(6):3092-3103. 26. Li T, Lindsley K, Rouse B, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: A systematic review and network meta-analysis. Ophthalmology. 2016;123(1):129-140. 27. Parrish RK, Palmberg P, Sheu WP, XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135(5):688-703. 28. Alm A, Villumsen J. PhXA34, a new potent ocular hypotensive drug. A study on dose-response relationship and on aqueous humor dynamics in healthy volunteers. Arch Ophthlmol. 1991;109(11):1564-1568. 29. Toris CB, Camras CB, Yablonski ME. Effects of PhXA41, a new prostaglandin F2, analog on aqueous humour dynamics on human eyes. Ophthalmology. 1993;100(9):1297-304. 30. Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008;53(S1):S107-20. 31. Fung DS, Whitson JT. An evidence-based review of unoprostene isopropyl ophthalmic solution 0.15% for glaucoma: place in therapy. Clin Ophthalmol. 2014;10(8):543-54. 32. Lee DA, Higginbotham EJ. Glaucoma and its treatment: A Review. Am J Health Syst Pharm. 2005;62(7):691-9. 33. Kopczynski CC, Epstein DL. Emerging trabecular outflow drugs. J Ocul Pharmacol Ther. 2014;30(2-3): 85-7. 34. Webster AR, Luff AJ, Canning CR, Elkington AR. The effect of pilocarpine on the glaucomatous visual field. Br J Ophthalmol. 1993;77(11):721-5. 35. Sihota R, Agarwal HC, Rajashekar YL. A comparative evaluation of pilocarpine 1% and clonidine 0.125% versus timolol 0.5%. Indian J Ophthalmol. 1996;44(2):87-9. 36. Burr J, Azuara-Blanco A, Avenell A, Tuulonen A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2012;(9):CD004399. 37. Alm A, Camras CB, Watson PG. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(Suppl 2):S105-110. 38. Aerie Pharmaceutical Products. http://aeriepharma.com/products-at-a-glance/. Accessed April 22, 2017. 39. Ren R, Li G, Le TD, et al. Netarsudil increases outflow facility in human eyes through multiple mechanisms. Invest Ophthalmol Vis Sci. 2016;57:6197–209. 40. Lua LJ, Tsaib JC, Liua J. Novel pharmacologic candidates for treatment of primary open-angle glaucoma. Yale Journal of Biology and Medicine. 2017;90:111-8. 41. Bacharach J, Dubiner HB, Levy B, et al. Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology. 2015;122(2):302-7 42. Perumal N, Manicam C, Steinicke M, et al. Characterization of the human aqueous humour proteome: A comparison of the genders. PLoS One. 2017;12(3):e0172481. 43. Kaufman PL. Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension. Expert Opinion on Pharmacotherapy. 2017;18(4):433-444. 44. Garcia GA, Ngai P, Mosaed S, Lin KY. Critical evaluation of latanoprostene bunod in the treatment of glaucoma. Clinical Ophthalmology. 2016 Oct;10:2035–50. 45. Krauss AH, Impagnatiello F, Toris CB, et al. Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating prostaglandin F2α agonist, in preclinical models. Exp Eye Res. 2011;93(3):250–55. |

