Three years ago, Douglas Devries, OD, of Sparks, Nev., was a guinea pig for a presbyopia-correcting eye drop that was in early development. At the time, Dr. Devries, who was on the drug’s advisory panel, had very low nearsightedness in one eye and no distance correction in the other eye. After he put the drops in, not only did his near vision improve, but his distance vision sharpened as well, he says. The clarity was so “phenomenal,” he drove around his Reno neighborhood to look at Christmas lights because his vision had never been better, Dr. Devries recalls.
“It was rather amazing. The effect lasted for over 12 hours. And it gave me probably the clearest distance vision I’ve ever had,” says Dr. Devries. The drop worked by creating a miotic pupil (pinhole) without the typical side effects of ciliary spasm or brow ache, and the miotic pupil eliminated most of the higher order aberrations resulting in very clear vision, he adds.
Today, patients with presbyopia may be one step closer to correcting their vision by eye drop, as several different research teams are investigating this alternative, noninvasive treatment that some optometrists believe could be a game changer.
“I see successful presbyopia correction as the ‘Holy Grail’ of vision correction surgery and something as simple as an eye drop as the icing on the cake,” says J. Christopher Freeman, OD, of Oklahoma City, Okla. “The need for presbyopic correction is a major point of visual dissatisfaction for patients, and to have nonsurgical options to eliminate the need for spectacle or contact lens correction would be a welcome addition to our presbyopia correction armamentarium.”
Here’s a snapshot of the new generation of presbyopia-correcting drops under development.
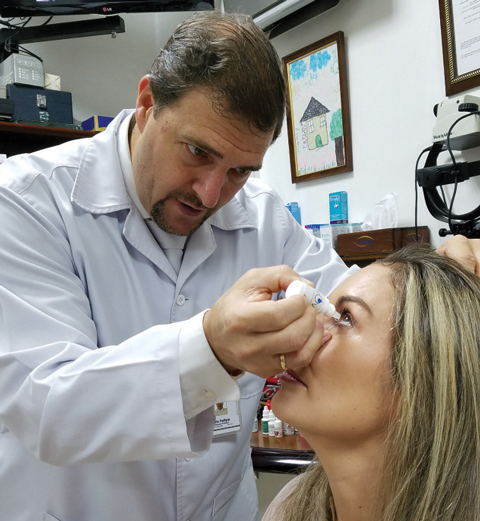 |
| Colombian researcher Felipe Vejarano, MD, a lead investigator for FOV Tears, instills the presbyopia-correcting drop into a patient’s eye. Initial study results suggest she could be completely independent of her near vision correction for normal activities after using the drops for three months. Photo: Felipe Vejarano, MD |
Reversing the Aging Eye
One promising new agent is EVO6 (Novartis), which is designed to restore crystalline lens flexibility and, hopefully, reverse the effects of aging in the eye.1
EV06 is a prodrug comprised of lipoic acid choline ester 1.5%, which penetrates the cornea and then breaks down into two naturally occurring substances: lipoic acid and choline, says Thomas Quinn, OD, of Athens, Ohio. The lipoic acid then converts to the active agent dihydolipoic acid, which breaks down disulphide bonds in the lens, improving flexibility, he says.
EV06 may potentially halt or reverse lens hardening and, in turn, would allow the lens to maintain or regain its ability to accommodate.1
In a Phase I-II masked, placebo-controlled proof-of-concept study, 50 patients were treated daily for 90 days with topical EV06 and 25 patients with placebo. EV06 showed a statistically significant difference from placebo in distant-corrected near vision at all time points measured (from day eight); at day 90, 82% of participants treated with EV06 had 20/40 near vision vs. 48% in the placebo group.2
The results are promising, as EV06 was well tolerated with comfort scores equivalent to the control drop, with no loss in best-corrected distance vision, Dr. Quinn says.
“Many of the other tools we currently employ to aid the presbyopic patient require the visual system to adapt to an imbalance (monovision) or simultaneously share multiple points of focus,” Dr. Quinn says. “Some brains deal with these optical adjustments just fine, while some struggle. With EV06, no such adjustment is required. Its optical effect is more like the natural focusing process we all are accustomed to early in life.”
While early results are positive, there are still many unknowns, as the study was small in scope, “so we must be cautious until we see results following use by a greater number of subjects,” says Dr. Quinn. “The big unanswered question is, ‘How long does the effect last?’ We won’t know that until studies currently underway are completed.”
Due to the simplicity of the optics it regenerates, EV06 may provide some exceptional visual benefits, according to Dr. Quinn. However, in the study, nearly 20% of subjects could not see at the 20/40 level after three months. “Would that improve after using the drop a few more weeks or months? We don’t know. If not, employment of one or more of the other options we now have at our disposal may be necessary,” Dr. Quinn adds.
Although some optometrists are optimistically cautious, others think EV06 could create an explosive new category for presbyopes.
“Really, this could potentially be a game changer,” says Mile Brujic, OD, of Bowling Green, Ohio. “This could open up a whole new world. It could ultimately change the way we think about treating presbyopia. Right now, we only know three-month results. In two or three years, we may find that the drop has the ability to alter the way the lens naturally ages.”
As patients age, there are certain expectations, including the need for cataract surgery, and lens hardening and presbyopia are only precursors to this, Dr. Brujic says. “So, by actually adding some functionality back to the lens, we are reversing some of the natural aging process. The eye care field needs to be prepped for this because it’s going to change the way we think about everything, and I think it’s going to change things in a really good way.”
The company expects pivotal phase III studies to begin in 2018 to evaluate long-term safety and duration of treatment, according to Dr. Freeman.
Finding a Niche in a Crowded FieldPresbyopes have myriad options, from tried-and-true readers, progressives and multifocal contact lenses to more invasive techniques such as IOLs and corneal inlays. If drops do make it to market, they could make significant waves in the presbyopia correction world. Working in Tandem Dr. Freeman also believes patients may still require some assistance above and beyond the drops, such as a low-power pair of reading glasses or a very weak monovision or multifocal contact lens for those with early presbyopia. “This could be quite an advantage for a patient to not progress past early presbyopia until around the time of cataract surgery,” he says. It’s too early to say how drops may affect various cataract forms. “Nuclear sclerosis cataract progression, for example, might be delayed. This might allow for a longer time-value of laser vision correction, such as LASIK,” bolstering that option, Dr. Freeman notes. Let’s Compare Drops would also eliminate many problems associated with spectacle lens wear. There wouldn’t be the limitation of only being able to see at near when looking through the lens, or frustrations of wearing them in situations where spectacles are inconvenient, such as outdoors, in inclement weather or for exercise, Dr. Freeman says. In addition, drops avoid the potential risks of a surgical procedure such as an IOL or a corneal inlay, Dr. Freeman says. “Different surgical options have different limitations, too, such as only one eye providing the near and intermediate vision with corneal inlays. And presbyopia-correcting IOLs may have varying levels of visual quality changes depending on lens type and which one is chosen,” Dr. Freeman says. Patients could use drops when they wanted without making a permanent, invasive choice. “I think that’s where the real appeal is. They try it, and if it works, great and then they continue. With a corneal inlay, that’s a lot bigger commitment,” Dr. Devries says. A Good Partner for Contact Lenses Still No “Magic Bullet” |
A “Lifestyle Drop”
Presbyopia Therapies is moving forward with development of its Liquid Vision drops, a temporary presbyopia-correcting therapeutic designed to last five hours or longer. Liquid Vision recently completed its Phase IIa trial that studied dosing, safety and efficacy, and a Phase IIb trial is expected to have preliminary results by the end of the year.
Liquid Vision eye drops combine aceclidine (a miotic) with tropicamide (a cycloplegic) to create a “super pinhole” effect and moderate accommodation, according to the company. Aceclidine is used for creating the pinhole effect (between 1.9mm and 1.5mm in pupil diameter). However, by itself this creates strong accommodation, including ciliary spasm and distance vision blur. Tropicamide moderates accommodation, and the ratio and interaction between these two drugs is required to improve both near and distance vision at the same time, Jim McCollum, cofounder of Presbyopia Therapies, says.
The drop is relatively fast acting, with a 30-minute onset after application, according to Mr. McCollum. The drop is a binocular treatment and is designed to improve near and distance vision, Mr. McCollum says. In the early days of development, the company initially concentrated on miotics that constricted the pupil but also created the unwanted ciliary side effects. To prevent this, Presbyopia Therapies added tropicamide to help moderate accommodation and reduce the associated distance vision blur.
The drops are intended for daily use and provide a “lifestyle enhancement” as a complement to current treatment options, not a permanent replacement of spectacles or contact lenses, according to Mr. McCollum. “The idea is, the patient uses the drops in the morning, drives to work, waits about 30 minutes for the drop to take effect, and then it will last half a day or longer. So if you’re working on the computer, you don’t need correction,” says Mr. McCollum. “If you need a second drop, you use a second drop. It’s a temporary solution by design.”
As Liquid Vision continues to move through FDA trials, the company hopes the drops will be on the market by 2021, according to Mr. McCollum.
The Great UnknownThe promise of presbyopia correction by drop sounds good to many on paper, yet there are still unanswered questions and a few perceived inconveniences. “The big question will be whether or not continued use will be required and thus continued expense over an extended period of time, or will a specific time of treatment be sufficient for correction, or even repeated courses of treatment, each for a certain amount of time,” Dr. Freeman says. Further research and cost analysis must better compare drops with the time-value of a surgical option, taking into consideration the risks and visual outcome of surgery, for quality comparison, he adds. Another unanswered question is which patients would make ideal candidates, Dr. Freeman says. “We’ll need to know more about comfort and tolerability as far as any adverse effects on the ocular surface. And in regard to the small-aperture optics of the bimonidine-carbachol drop, ocular scatter, especially as it related to any lenticular opacities, could be a limiting factor. The big advantage is that if it doesn’t work, the patient simply stops using the eye drop, as long as no long-term detrimental effects are discovered.” And while drops offer a noninvasive solution to presbyopia correction, some patients may prefer the usual standbys of readers or progressives to avoid putting a drop in their eye. |
Correction Buildup
FOV Tears, a binocular presbyopia-correcting drop, is now available in Colombia and is working its way through the approval pipelines in Argentina, Peru and Spain.
FOV Tears is a combination of a parasympathetic, alpha agonists 1 and 2, an anti-cholinesterase and an NSAID, according to Colombian researcher Felipe Vejarano, MD, a lead investigator for the drug.
The drop affects the ciliary muscle, which causes a physiological accommodation and a dynamic pseudo-accommodation. “This means a mild and dynamic miosis, which changes with light intensity.”
A recent follow-up to a pilot study tracked patients who used the drop for three months.3 Although small—only 14 emmetropic presbyopic subjects with an average age of 55—the study provided some promising results. The study found that while the duration of the drop’s effect lasted four to five hours initially, duration increased to eight hours if the patient used the drop over time, says Dr. Vejarano. The onset of the drop’s effects also accelerated with continual use, he says. While 25% of study participants said the drop took five to 10 minutes to take effect in week one, roughly 70% said it took only five to 10 minutes by the third month.3 By the third month of use, 100% of participants said they were totally independent of their near vision correction for normal activities.3
Initial research found the drops improved near vision by two to three lines.3 After three months, they found near vision improved by an additional one to two lines. Results showed distance vision increased by one line, and study participants had intermediate vision of 20/25 or better, according to Dr. Vejarano.
The initial research showed a few additional benefits, such as a decrease of 1mm Hg in patients’ intraocular pressure with continued use.3 The patient satisfaction survey also revealed patients felt the drops improved the uncomfortable sensation associated with dilation.3
Combination Correction
Researchers are also investigating a carbachol and brimonidine drop for presbyopia correction. In a recent pilot study, 10 naturally emmetropic and presbyopic subjects between the ages of 42 and 58 with uncorrected distance visual acuity of at least 20/20 in both eyes received 3% carbachol and 0.2% brimonidine in both combined and separate forms, 3% carbachol alone and 0.2% brimonidine (control) alone in their non-dominant eye.4 Researchers found statistically significant improvement in mean near visual acuity in all subjects who received combined 3% carbachol and 0.2% brimonidine in the same formula compared with those who received separate forms, carbachol alone or brimonidine alone (p < 0.0001).4
The monocular pharmacologic treatment of presbyopia with one drop a day of carbachol/brimonidine in the non-dominant eye permits acceptable reading vision for many presbyopes, even older subjects, the researchers concluded.4
This drop constricts the pupil and increases depth of focus with the pinhole effect, similar to the Kamra corneal inlay (AcuFocus), according to Dr. Freeman. The disadvantage, he says, “is that it is only in one eye and the potential for reduced quality of vision or dimness sometimes is experienced with small-aperture optics.” However, many patients are happy with their improved near and intermediate vision following Kamra implantation, with the dominant eye usually masking perceived dimming monocularly in the non-dominant eye, he adds.
“The difference [with Kamra] is, the patient’s not paying for and using eye drops every day to enjoy that vision,” adds Dr. Freeman. “Improvement could be significant enough that a patient may elect this treatment over wearing spectacles or contact lenses if they are highly motivated not to wear contacts or glasses.”
Despite the small number and the heterogeneity of the patients involved in this pilot study, its findings suggest this drop is also promising.4 Additional studies are planned with hyperopic and myopic presbyopes and in pseudophakic and post-LASIK presbyopes.4
 |
| This image depicts the effect of a traditional miotic drug, pilocarpine, with pupil size at roughly 2.3mm. The drop provides some effect, but not a pinhole effect. Accommodation is achieved, but distance vision blur is present. Image: Presbyopia Therapies |
 |
| This image shows the results of a Liquid Vision drop. Aceclidine provides a stronger pinhole effect with the pupil less than 2mm. Tropicamide modulates the accommodation. Proponents say the combination/ratio of both drugs allows for improved near and distance vision at once, without typical side effects. Image: Presbyopia Therapies |
Opportunity for Everyone
“I look at this as a remarkable opportunity,” Dr. Brujic says. “My philosophy is very simple when it comes to an eye care practice. Do everything that is in the best interest of the patient. “With a drop that actually helps presbyopic symptoms, we can reduce dependency on presbyopic correction such as reading glasses and progressives.”
Presbyopia-correcting drops could also open up a door to new patients who thought they only needed over-the-counter reading glasses, Dr. Brujic says. “Now we’ll have the opportunity to help these patients because they’re going to be coming in asking if they are candidates for the drop.”
Patient interest in this advancement is “intense,” Dr. Devries says. “I still have patients who say, ‘You told me about a drop a few years ago that would let me see up close. Is it on the market yet?’ So they continue to ask. I think the relevance of a presbyopia drop to patients is going to be extreme.”
1. Encore Vision Announces Successful Phase I-II Study of Topical EV06 for the Treatment of Presbyopia. PR Newswire. May 5, 2016. www.prnewswire.com/news-releases/encore-vision-announces-successful-phase-i-ii-study-of-topical-ev06-for-the-treatment-of-presbyopia-300263690.html. Accessed May 1, 2017. |

