The choroid is the main source of blood to the eye and, as such, understanding its health can play a vital role in identifying and managing various ocular diseases. Traditionally, however, visualizing the choroid has been a near-impossible challenge, due to its positioning between the retinal pigment epithelium (RPE) and the sclera. This difficulty extends to the structures within the choroid, such as the choriocapillaris, a rich vascular supply that lies at the inner side of the choroid in a single plane beneath the retina. The outer layers of the choroid mainly consist of intermediate-sized vessels adjacent to the capillaries (Sattler’s layer), followed by larger vessels (Haller’s layer). The choriocapillaris is the main layer that provides nutrients to the outer retina, including the photoreceptor and RPE cells. Its integrity is crucial for visual function.
Luckily, these previously hidden structures are no longer out of optometrists’ reach thanks to the latest imaging modalities. This article explains how these technologies can be used to better evaluate the choroid.
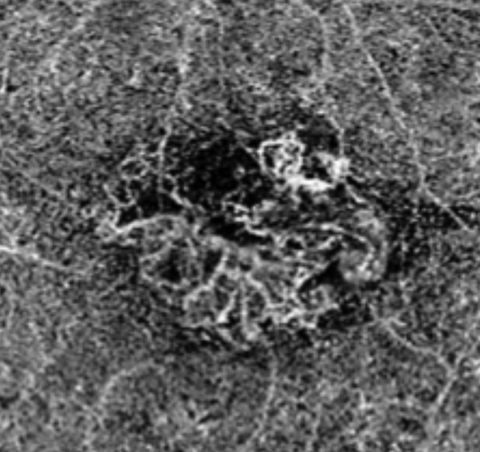 |
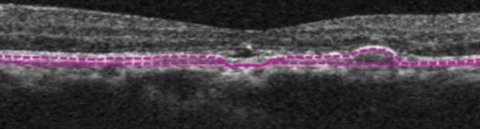
| Above, this segmentation image of the choroid, obtained via OCT-A, demonstrates the hyper-flow “sea fan” pattern. Below, this structural OCT image of the same eye shows RPE elevations and mild foveal subretinal fluid. Click images to enlarge. |
 |
Yesterday’s Limitations
Indocyanine green angiography (ICGA) has traditionally been used to study choroidal circulation, but it does not allow three-dimensional visualization of the individual layers.1,2 Conventional spectral-domain OCT (SD-OCT), while useful to visualize retinal layers with high resolution, is somewhat weaker in assessing the choroid, due to light scattering by the RPE layer, which results in decreased signal penetration through the retina into the choroid. This decreases the resolution at the choroid and visibility of the choroid-sclera interface. Although B-scan ultrasonography is helpful for visualizing some tumors and other features, it does not provide sufficient resolution to assess changes in choroidal thickness.
Today’s Improvements
Advanced imaging modalities, such as enhanced-depth imaging optical coherence tomography (EDI-OCT), allow for the visualization of deep structures, including the choroid and the choroidal-sclera interface, while still retaining retinal details. EDI uses SD-OCT with the peak sensitivity placed posteriorly, towards the sclera.3 It can penetrate up to 800µm and significantly improves the visualization of the choriocapillaris and choroidal vasculature, due to the proximity of these layers to the RPE.3 Thanks to EDI-OCT’s increased sensitivity in detecting the proper choroidal-sclera interface, we finally have a method by which we can measure choroidal thickness. Using this technology, researchers have found that the mean subfoveal choroidal thickness is approximately 287µm (plus or minus a standard deviation of 75.7µm).4
Knowing this has helped not only in the assessment of choroidal conditions, but also in the evaluation of the relationship between choroidal thickness and other ocular conditions. For example, we now know choroidal thickness typically decreases by approximately 16µm per decade of life.4
Choroidal thickness is now implicated in numerous retinal diseases, including age-related macular degeneration (AMD), adult-onset foveomacular dystrophy (AOFMD), central serous choroidopathy (CSC), diabetic retinopathy and retinitis pigmentosa, as well as in glaucoma. Choroidal thickness can also be affected by other factors, including diurnal variations, caffeine consumption and water intake.5 These changes are less prominent and usually don’t exceed 30µm.6
The ability to see further into the choroidal structures using EDI-OCT has become increasingly helpful in assessing conditions such as choroidal nevi, choroidal melanomas and choroidal neovascularization. However, even when using EDI-OCT, direct visualization of choroidal vessels and assessment of the choroidal vascular density remain a challenge.
The newest technology, OCT- angiography (OCT-A), provides a detailed vascular map of the posterior segment. OCT-A uses motion contrast to construct detailed volumetric angiographic images, mapping the retinal and choroidal vasculature in a matter of seconds. To assess blood flow (erythrocytes), it compares decorrelation signal (differences in the backscattered OCT intensity) between sequential OCT B-scans, taken at the same cross-section.3 It is a noninvasive imaging technique that has many potential applications in retinal and choroidal vascular diseases as it provides structural vascular information as well as functional blood flow information without the use of intravenous dye.
Our understanding of the choroid has vastly increased with the introduction of these imaging modalities. They have allowed the identification of conditions such as age-related choroidal atrophy (ARCA), pachychoroid and better assessment of choroidal neovascular membranes.
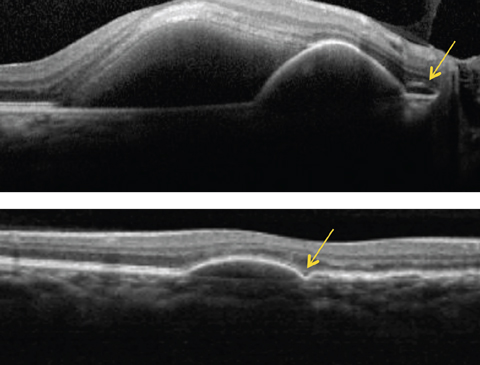 |
| Above, this SD-OCT shows a PCV patient. Note the dome-shaped elevation of RPE with an adjacent double layer sign (see arrow) and a serous retinal detachment. Below, this SD-OCT shows a different patient with PCV demonstrating a PED with a bola sign (see arrow). Click image to enlarge. |
Disease Types
Choroidal neovascular membranes (CNVMs) are characterized by the presence of abnormal blood vessels that originate from the choroid and extend either to the area between Bruch’s membrane and the RPE (as seen in type I), or into the subretinal space (as seen in type II). Funduscopic signs associated with CNVM include hemorrhages, retinal thickening, exudation and fibrosis, which often lead to photoreceptor damage and vision loss.7
CNVMs on OCT will generally appear as an area of hyper-reflectivity either below or above the RPE, depending on the type.7 Other OCT features associated with CNVMs, such as RPE elevation and accumulation of fluid at various levels of the retina, provide invaluable information in the assessment of the activity of the lesion and for its clinical management.
Not all membranes leak, nor does leakage always signify the presence of a concomitant CNVM. For example, a CNVM secondary to pathological myopia, angioid streaks, histoplasmosis, CSC or inflammatory disease tend to have minimal leakage on traditional fluorescein angiography (FA). Furthermore, early changes may also go undetected and, although research shows SD-OCT and EDI-OCT are beneficial in the detection of CNVM, various entities may have similar location and reflectivity, which may cause challenges in interpretation.7 The inability of these techniques to accurately visualize the lesion or its perfusion results in decreased sensitivity and specificity in the diagnosis of a CNVM.
Traditional angiography, FA or indocyanine green angiography (ICGA), remain the standard method of evaluating choroidal conditions, particularly for the evaluation of CNVM; however, newer methods have some major improvements. OCT-A, for instance, is dyeless and provides high-resolution imaging of the choroidal vasculature, enabling it to delineate the complex vasculature associated with a CNVM. Studies show that the sensitivity of OCT-A in detecting CNVMs when compared with FA ranges from 50% to 100%.8-10 On the other hand, the sensitivity of CNVM diagnosis in diseases such as CSC and in cases of exudative AMD may be as high as 100% and 80%, respectfully.11,12 The difference in sensitivity between conditions is probably due to the fact that an overlying massive hemorrhage is more likely to occur in AMD, as opposed to other conditions such as chronic CSC. The hemorrhage will block the signal and limit the visualization of CNVMs.9,13
OCT-A features associated with CNVM may include patterns described as small filamentous vessels forming anastomoses (lacy wheel or sea fan), as well as vessels associated with a central trunk (Medusa).11,13 In contrast to FA, OCT-A allows a three-dimensional visualization of the fibrovascular network, by providing high-resolution depth-encoded images without any hindering effect associated with dye leakage. OCT-A is therefore invaluable in the qualitative assessment of CNVM, including its multi-planar location and morphology. This may aid in future assessment of morphological changes associated with treatment response.
Limitations of OCT-A include hindering effects from various artifacts, including projection artifacts which occur due to shadows created by moving erythrocytes in more superficial vessels, giving the false impression of blood flow in deeper layers. Another limitation is that OCT-A will detect a blood vessel only if its blood flow speed is higher than a minimum threshold, which is determined by the time between two sequential OCT B-scans.14 CNVMs may show areas of reduced blood flow, potentially making them completely or partially undetectable on OCT-A.
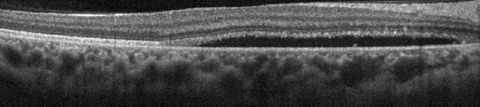 |
| This SD-OCT shows a CSC patient demonstrating relatively thickened choroid (pachychoroid) with dilation of large choroidal vessels (pachyvessels) and relative absence of choriocapillaris and Sattler’s layer in the area underlying the subretinal fluid. Click image to enlarge. |
The pachychoroid spectrum encompasses a multitude of macular disorders that share similar features, including increased choroidal thickness, dilation of the large choroidal vessels (Haller’s layer) or “pachyvessels” and loss of choriocapillaris/Sattler’s layer overlying the pachyvessels due to their compression by the latter.15-17 The spectrum includes pachychoroid pigment epitheliopathy (PPE), CSC, pachychoroid neovasculopathy (PNV) and polypoidal choroidal vasculopathy (PCV). The classic and unifying feature for these conditions is the assessment of an increased choroidal thickness (pachychoroid), best evaluated through the use of EDI-OCT. This feature is helpful in the differential diagnosis of conditions within the pachychoroid spectrum from AMD.
Pachychoroid pigment epitheliopathy, researchers believe, is a precursor of CSC. It’s characterized by the presence of RPE changes with absence of preceding or concurrent neurosensory detachment.16,17 These RPE changes have similar appearance to findings often seen in the fellow eye of patients with unilateral CSC and may be mistaken for AMD changes. These include features associated with RPE hyperplasia and drusen-like deposits.17 Classically, a thickened choroid is denoted on EDI-OCT, which is due to pachyvessels, with overlying attenuation of the choriocapillaris and Sattler’s layer.18 The pachyvessels, which run in close proximity to the RPE-Bruch membrane complex, incite pigment epitheliopathy changes.18
Central serous choroidopathy is characterized by an idiopathic serous neurosensory detachment often associated with serous pigment epithelial detachments (PEDs) and no evidence of inflammatory features or presence of a CNVM. SD-OCT shows a typical smooth and convex profile of neurosensory detachment with underlying hypo-reflectivity. EDI-OCT shows a thickened choroid and localizes the pachyvessels observed on ICGA to the outer choroid. This may help in the differential diagnosis with subretinal fluid associated with wet AMD. Chronic CSC (longer than three months) may result in RPE changes, thickened photoreceptor outer segments, ellipsoid zone disruption and, eventually, outer retinal atrophy, neovascularization formation (PNV), or both.16
Pachychoroid neovasculopathy is characterized by the presence of type I CNVM associated with a pachychoroid phenotype, such as a chronic CSC. The presence of shallow irregular RPE elevations on SD-OCT in eyes with pachychoroid should raise suspicion for neovascularization. PNV is distinguished from neovascular AMD by several features, including younger age at onset of neovascularization, a relative absence of drusen, and a thick choroid with pachyvessels as seen on EDI-OCT.16 On the other hand, patients older than 60 years may exhibit some clinical features common to both PNV and neovascular AMD but with thinning of the choroid. Combining both EDI-OCT—to evaluate choroidal thickness—and OCT-A—to determine the presence of a CNVM—is critical in the diagnosis of PNV.
Polypoidal choroidal vasculopathy can be confirmed by the presence of polypoidal lesions with or without branching network on ICGA. Clinical findings include recurrent serosanguinous PEDs and may mimic those associated with wet AMD. Serous retinal detachments are often present. Two distinct signs are observed on OCT, the “double layer” sign described as two hyper-reflective lines within the vicinity of the PED, representing the branching vascular network, and the “bolas sign” described as an RPE disruption, representing a small polyp adjacent to the PED.19-21 PCV is highly suspect if funduscopic findings are noted in adults of Caribbean, Asian or African descent.22-24 Unlike with wet AMD, EDI-OCT of a PCV will show a pachychoroid. Other distinguishing features on structural OCT include the increased height of the serous retinal detachment, which also occurs in higher frequency, and less intraretinal edema.22-24 Although ICGA remains the standard to diagnose of PCV, OCT-A may help to identify the PCV complex. Using segmentation of the choriocapillaris, the branching vascular network will appear as a hyper flow lesion whereas the polypoidal lesion will demonstrate lower flow, and will appear either as a hyper flow round structure surrounded by a hypo-intense halo, or more frequently, as a hypo flow round structure. The lower flow of the polypoidal lesion is likely due to unusual blood flow within it in contrast with the branching vascular network.25
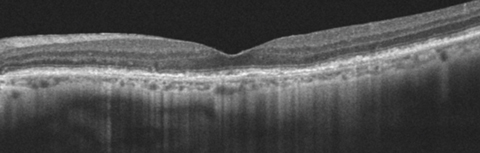 |
| This 77-year-old patient’s OCT demonstrates ARAC. The PIL line is intact with slight RPE mottling. Click image to enlarge. |
Age-related choroidal atrophy is a relatively newly described entity in patients older than 60 years with decreased visual acuity where the primary abnormality seems to be in the choroid.26 It is characterized by markedly decreased choroidal thickness under the fovea and particularly nasal to the fovea, along with pigmentary changes and a rarefaction of visible choroidal vessels under the macula. In fact, since the choroid, and more specifically the choriocapillaris, plays a vital role by providing nutrients to photoreceptors and RPE cells, its decreased perfusion should lead to ischemic progressive photoreceptor and RPE cell death in the foveal area, eventually leading to decreased visual acuity. One study of 17 patients shows subfoveal choroidal thickness in ARCA is less than 125µm, with an average of 69.8µm.26 However, this cut-off of 125µm of choroidal thickness does not represent an absolute demarcation.26
The main differential diagnosis with ARCA is AMD. Pathological features of AMD include drusen and pigmentary changes eventually progressing into geographic atrophy due to RPE and photoreceptor death. AMD itself has been linked with choroidal thinning and reduced vascular density, especially in the advanced stage of the disease. However, the differentiating characteristic of ARCA with AMD is that the main abnormality correlating with the decreased visual acuity seems to be severe choroidal thinning as opposed to the presence of geographic atrophy or changes characteristic of wet AMD.26 In addition, the funduscopic presence of drusen are also not a common finding with ARCA but rather with AMD.26 EDI-OCT is helpful in determining the thickness of the choroid, and therefore in the differential diagnosis.26
Our understanding of choroidal diseases has been augmented in recent years with the advent of advanced imaging techniques: EDI-OCT and OCT-A. These techniques allow for noninvasive, improved qualitative and quantitative analysis of the choroid and are invaluable in the diagnosis and management of a variety of chorioretinal diseases. Incorporating these technologies also opens the doors to newer and more disease-specific treatment modalities.
Dr. Makhlouf is an assistant professor with a focus on primary eye care and ocular disease at Nova Southeastern University College of Optometry in Fort Lauderdale, Fla. She teaches the course in ophthalmic lasers and surgical comanagement.
Dr. Shechtman is a professor of optometry at Nova Southeastern University College of Optometry.
Dr. Reynolds is an associate professor of optometry at Nova Southeastern University College of Optometry.
1. Stanga P, Lim J, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003;110:15–21. |

