 |
Autologous serum (AS) tears or autologous serum eye drops (ASEDs) are customized drops made from a patient’s own blood diluted in sterile saline or hyaluronic acid. Because serum is composed of a complex mix of growth factors, proteins, antioxidants and lipids, it is more akin to the components of human tears and may provide a more effective replacement than manufactured tears. The lacrimal gland uses serum components to generate tear fluid, so it makes intuitive sense that, in patients with malfunctioning lacrimal glands, we might be able to mimic that function by using serum in topical form.
The first use of AS tears was reported in 1975, and for the next 25 years or so, they were considered a last resort and used only by corneal specialists.1 However, with today’s advancements, they have been embraced by a wider range of clinicians and are being used earlier in the course of dry eye disease.
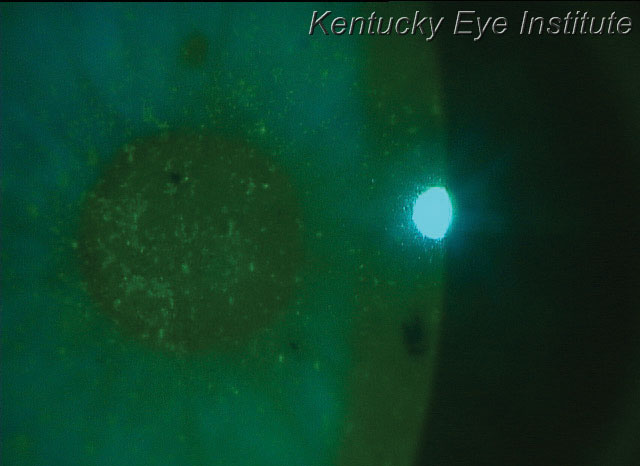 |
| New autologous serum options make it more feasible for treating conditions such as superficial punctate keratitis, seen here in a patient with keratoconjunctivitis sicca. |
Evidence for AS tears
Research shows AS tears are more effective than conventional tears for improving tear film stability and subjective comfort in patients with severe dry eye.2 They also provide statistically significant improvements in Schirmer’s scores, tear film debris and goblet and epithelial cell density.3 In a California Kaiser Permanente population of mostly recalcitrant dry eye patients treated with AS tears as an insurance-covered benefit, patients had improved corneal staining and reduced dependence on artificial tears.4
AS tears are also effective at improving both the signs and symptoms of ocular surface disorders associated with systemic autoimmune diseases. In about 80% of patients with conditions such as Sjögren’s syndrome (SS), mucous membrane pemphigoid, graft-vs.-host disease (GVHD), rheumatoid arthritis and other autoimmune disorders, the researchers demonstrated improvement in corneal staining, reduction in punctate epithelial erosions and persistent epithelial defects and improvements in subjective symptoms.5
This treatment modality can also accelerate corneal epithelial healing after surface ablation corneal refractive surgery and pterygium surgery.6,7
ASED FactsThe serum concentration is most commonly 20% for dry eye disease patients, although higher concentrations of 40% or even 50% are often used in advanced conditions such as neurotrophic corneal ulcers. The drops are usually dosed anywhere from two to eight times per day, with six or Q2H being the most common, for the treatment of aqueous-deficient dry eye, neurotrophic keratitis or ocular surface disease secondary to systemic autoimmune syndromes. |
Adoption Hurdles
Historically, challenges with AS tears have hindered their use. A recent Cochrane review of randomized controlled trials in which AS tears were used to treat patients with SS, non-SS dry eye and postoperative dry eye found inconsistencies in the literature and concluded that current evidence did not necessarily support a benefit; but the study data was based on only two weeks of treatment.8 Limited studies were available for review, and the authors acknowledged that much more research is still needed.
Beyond scarce support, the modality poses patient-specific concerns. Patients must not have any blood-borne infectious conditions and have their blood drawn regularly. Once compounded, ASED vials must be kept frozen or refrigerated to avoid contamination, degradation of the components or both. Additionally, AS tears can be expensive and are not always covered by insurance.
Changing Paradigms
But as optometrists become more effective and more aggressive in treating ocular surface conditions, AS tears are rising in popularity. The International Task Force Delphi Panel on Dry Eye identified ASEDs as a recommended treatment for patients with level three disease, as opposed to only for level four (the most severe cases).9
Beyond the therapy’s clinical benefits, advances in the technology have been another major driver to the increased use of ASEDs. The advent of self-retained cryopreserved amniotic membrane (Prokera, BioTissue) has put ASEDs in a far more accessible package for in-office use. In my practice, for example, we move quickly to amniotic membrane for patients with persistent superficial punctate keratitis, recalcitrant recurrent corneal erosion, neurotrophic keratitis and neuropathic keratitis. We have even use it in earlier stages of dry eye with decreased vision, and almost always prescribe ASEDs long-term in conjunction with amniotic membrane.
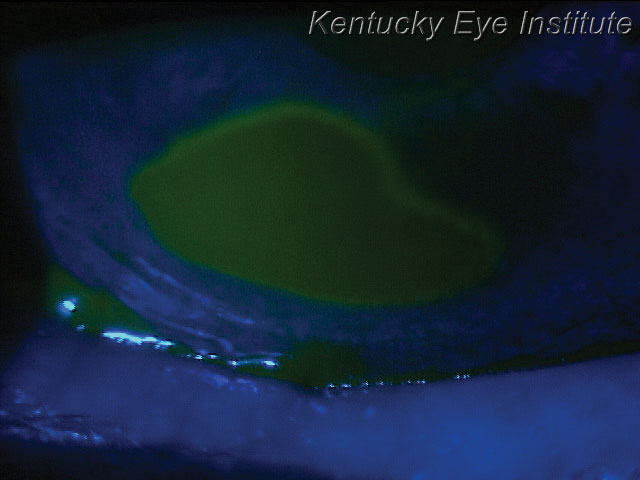 |
| A neurotrophic ulcer, such as this one, can be treated quickly with an amniotic membrane to help promote healing. |
Additionally, AS tears have become much more accessible through compounding pharmacies and eye banks. Recently, Vital Tears in Kansas City, Mo., has become a nationwide source for ASEDs. The company works with a network of facilities for local blood collection and coordinates production, quick delivery and billing. The drops are made to clinician specifications and available to patients on a short-term or ongoing subscription basis.
Other new approaches are in the pipeline as well. A biologic eye drop (Elate Ocular, Cambium Medical Technologies) for dry eye is currently in Phase II clinical trials. These are allogeneic, rather than autologous, human platelet-derived topical drops expected to be shelf-stable, eliminating the need for refrigeration. Sourcing serum from healthy young donors for these allogeneic serum tears may avoid elevated proinflammatory cytokine levels in the serum of patients with active autoimmune diseases.
Drops derived from umbilical cord serum—avoiding issues with donation of autologous serum—is also under investigation.10 Anecdotally, many patients with advanced ocular surface disease and dry eye disease have achieved significant relief with the use of commercially available amniotic cytokine extract (ACE) eye drops. Genesis drops (Ocular Science) BID have the potential to modulate corneal and conjunctival epithelial healing, as well as inflammation. Other researchers are investigating the use of platelet-rich plasma AS, which uses a portion of the patient’s own blood that has a higher-than-normal platelet concentration, with the goal of creating a prolonged release of growth factors that are involved in the wound healing process.11
Serum-derived and biologic-based eye drops are poised to make waves in our clinical approach to dry eye patients, and new options continue to crop up. In patients who can’t make enough of their own tears because of advanced keratoconjunctivitis sicca, GVHD, SS and numerous other ocular surface diseases, the current therapies are essential—and are getting better by the day.
Dr. Karpecki is a consultant/advisor to: BioTissue, Cambium Medical Technologies, Vital Tears and Ocular Science.
1. Fox RI, Chan R, Michelson JB, et al. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984;27(4):459-61. 2. Celebi AR, Ulusoy C, Mirza GE. The efficacy of autologous serum eye drops for severe dry eye syndrome: A randomized double-blink crossover study. Graefes Arch Clin Exp Ophthalmol 2014;252(4):619-26. 3. Jirsova K, Brejchova K, Krabcova I, et al. The application of autologous serum eye drops in severe dry eye patients; subjective and objective parameters before and after treatment. Curr Eye Res 2014;39(1):21-30. 4. Dalmon CA, Chandra NS, Jeng BH. Use of autologous serum eyedrops for the treatment of ocular surface disease: First US experience in a large population as an insurance-covered benefit. Arch Ophthalmol. 2012;130(12):1612-3. 5. Ali TK, Gibbons A, Cartes C, et al. Use of autologous serum tears for the treatment of ocular surface disease from patients with systemic autoimmune diseases. Am J Ophthalmol. 2018;189:65-70. 6. Akcam HT, Unlu M, Karaca EE, et al. Autologuous serum eye-drops and enhanced epithelial healing time after photorefractive keratectomy. Clin Exp Optom. 2018;101(1):34-7. 7. Sul S, Korkmaz S, Alacamli G, et al. Application of autologous serum eye drops after pterygium surgery: A prospective study. Graefes Arch Clin Exp Ophthalmol. July 18, 2018. [Epub ahead of print]. 8. Pan Q, Angelina A, Marrone M, et al. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. February 28, 2017. 9. Behrens A, Doyle JJ, Stern L, et al; Dysfunctional tear syndrome study group. Dysfunctional tear syndrome: A Delphi approach to treatment recommendations. Cornea. 2006;25:900-7. 10. Yoon KC, Heo H, Im SK, et al. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol 2007;144(1):86-92. 11. Alio JL, Rodriguez AE, Wrobel Dudzinska D. Eye platelet-rich plasma in the treatment of ocular surface disorders. Curr Opin Ophthalmol. 2015;26(4):325-32. |

