 |
Imaging technology continues to advance at a fast pace. While the advent of new tools and techniques enhances our current practices and diagnostic abilities, we should be cautious not to lose our appreciation for some of the fundamental options that we already have at our disposal. Monochromatic filters are simple and readily available tools that are often taken for granted.
Filter Out Your Options
When white light is used to view the eye and its contents, we are able to examine the ocular tissues in their normal state and appreciate their natural colorful hue and saturation. This is very much a necessity, especially when assessing for subtle pallor of the neuroretinal rim. However, during a funduscopic exam, our ability to diagnose certain ocular findings is often limited by the lack of contrast between the area of concern and the surrounding tissue due to their seemingly similar coloration. A monochromatic filter greatly improves the contrast and resolution of the tissues in question, allowing for better localization and accurate diagnosis.1
There are several well-known monochromatic filters used on ophthalmoscopic examination. For example, the cobalt blue filter is frequently used, as it provides the necessary wavelength of illumination to excite fluorescein and better visualize the mires when performing Goldmann applanation tonometry. It is the often-underused red-free filter, however, that may be the most versatile.
The red-free filter, or “green” filter, is an available illumination setting on most modern slit lamps, direct ophthalmoscopes, binocular indirect ophthalmoscopes and fundus cameras. Longer wavelengths on the red end of the visible spectrum are blocked by this filter when it is applied to white light.2 Essentially, this filter blocks out the visual “noise” and allows us to see greater contrast between the structures and tissues. This is especially significant on fundus examination; the predominant color of the retinal background is a reddish hue.
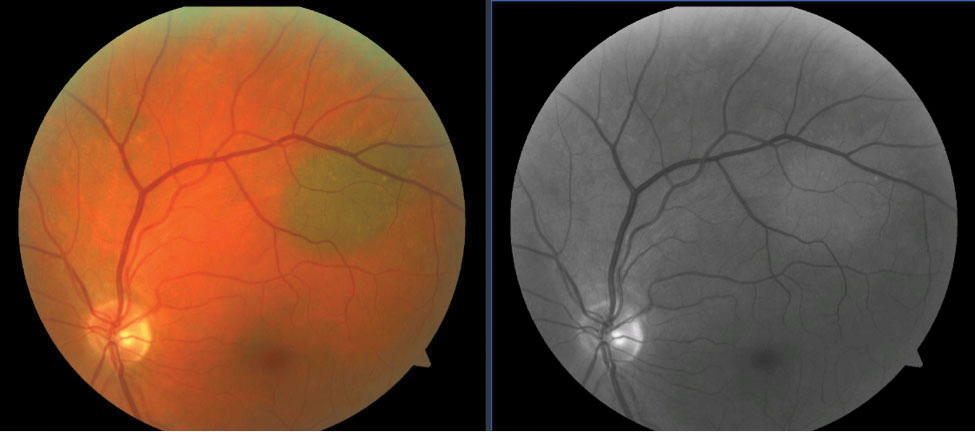 |
| Fig. 1. A choroidal nevus is seen superior-temporal to the optic nerve (left). Choroidal lesions disappear or become much lighter with use of the red-free filter as blood vessels appear black against the uniformly dark background caused by the retinal pigment epithelium (right). Click image to enlarge. |
The most commonly used red-free filters have a peak transmission of 540nm to 570nm in wavelength, allowing for observation of the greenish color that is seen when the filter is used in a slit lamp or other illumination device.3 The utility of the filter extends beyond improving retinal contrast; in fact, every area of the eye, from anterior to posterior structures, can benefit in some way from examination with this multifaceted filter.
Anterior. Beginning with the outer segment of the eye, the red-free filter can enhance intraocular pressure (IOP) measurements. The gold standard for IOP measurement, Goldmann applanation tonometry, uses cobalt blue light to accompany the instilled fluorescein dye. Alternatively, studies have suggested that IOP measured with the red-free filter and a topical anesthetic also yields accurate results.4 For many patients, this method was better tolerated and more comfortable than traditional blue light with fluorescein and reduced the risk of accidental spillage with fluorescein dye and stained clothing.4 It is also worth considering if topical fluorescein cannot be procured or if there is reason to suspect a fluorescein allergy.
Another potential anterior segment use of red-free filters is in the case of urgent or emergent presentations of red eyes. The filter causes blood vessels and blood to appear darker—almost black—enhancing contrast.3 When differentiating between similar conditions, such as episcleritis and scleritis, an accurate and timely diagnosis is crucial to employ the appropriate treatment and management plan. This important distinction can be made by applying the red-free filter to improve localization of the congested vessels and depth of inflammation.5
In the same manner, the red-free filter may also be employed in cases of traumatic microhyphema. It is often difficult to identify small areas of bleeding with a dark iris as a backdrop. Accentuating red blood cells can also help distinguish between pigmented cells that may be a remnant of old inflammation and active inflammatory white blood cells when viewing the anterior chamber. In such cases, pigmented cells within the anterior chamber disappear, while active white blood cells remain visible.5
Posterior. Where the red-free filter really shines, however, is in the back of the eye. To fully appreciate its value, we must understand the light dynamic and the retina. The visible spectrum is divided into short (blue), intermediate (green) and long (red) wavelengths. Shorter wavelengths are heavily scattered by the ocular media and primarily impaired by changes in the eye, such as cataracts. The blue light unable to be transmitted to the retina is reflected by the internal limiting membrane and the additional anterior retinal layers to provide good visualization.1 For this reason, a blue filter is a good option when attempting to photograph anterior retinal pathology, such as fibrovascular membranes in cases of proliferative diabetic retinopathy or epiretinal membranes.
Green wavelengths are not as heavily scattered by the ocular media, meaning that more of this light reaches the back of the eye. Green, or red-free, light is better suited to visualize the retinal nerve fiber layer (RNFL), especially when cataracts are present.3 To some extent, green light is reflected by a healthy, intact RNFL. If there has been localized damage to the RNFL from glaucoma, a darker wedge will be seen juxtaposed between the normal healthy RNFL striations. There is good correlation between RNFL defects seen with the red-free filter and with thinning of the RNFL seen on OCT.6 The enhanced contrast offered by the red-free filter can also assist in more accurately assessing the cup-to-disc ratio, especially in the case of a shallow cup.7
Green wavelengths have the added benefit of penetrating into the deeper retinal layers. Most of the transmitted green light is reflected by melanin in the retinal pigment epithelium, resulting in a uniformly dark background best for visualizing retinal pathology.2 Generally, if a naturally appearing red-hued structure is viewed through a red wavelength filter, it will appear lighter. However, if the same structure is viewed through the contrasting green filter, it will appear black. By this principle, blood vessels absorb green light and appear black because of the hemoglobin they contain.
Green light at 570nm is the ideal wavelength to view blood vessels and evaluate their caliber, crossing changes or the presence of pathology.1 For this reason, there is a risk of underestimating certain types of retinopathy, such as that which occurs with diabetes or hypertension where small hemorrhages are a common finding.8 Modern fundus cameras use a grayscale color scheme for their blue, green and red wavelength filters. This also serves to increase the resolution, given that every pixel is dedicated to the grayscale and decreases chromatic aberrations.
Green wavelengths do not penetrate into the choroidal layer. This explains the well-known phenomenon of choroidal lesions seemingly disappearing when the red-free filter is applied. Choroidal nevi are the most common intraocular tumors, and though they are benign, they require close monitoring for conversion to possible malignant melanoma.9 Red-free fundus imaging is an essential piece of the initial documentation and continued monitoring of a choroidal nevus (Figure 1).
Takeaways
The red-free filter has a lot to offer optometrists in both routine and urgent or emergent care. Its primary advantage is the increased contrast it offers between the ocular structures under examination and their vasculature. Some of its applications are well-known to clinicians and frequently used. Others, however, are routinely underused and could play a helpful role in the diagnosis of a variety of conditions.
Dr. Leburg is an instructor at the Pennsylvania College of Optometry, where she completed a residency in primary care/ocular disease in 2017. She divides her time between precepting interns at the Eye Institute and teaching in the clinical skills program at Salus University. She has no financial interests to disclose.
Dr. Labib graduated from Pennsylvania College of Optometry, where she now works as an associate professor. She completed her residency in primary care/ocular disease and is a fellow of the American Academy of Optometry and a diplomate in the Comprehensive Eye Care section. She has no financial interests to disclose.
1. Delori FC, Gragoudas ES. Examination of the ocular fundus with monochromatic light. Ann Ophthalmol. 1976;8(6):703-9. 2. Delori FC, Pflibsen KP. Spectral reflectance of the human ocular fundus. Appl Opt. 1989;28(6):1061-77. 3. Ducrey NM, Delori FC, Gragoudas ES. Monochromatic ophthalmoscopy and fundus photography. II. The pathological fundus. Arch Ophthalmol. 1979;97(2):288-93. 4. Ghoneim EM. Red-free light for measurement of intraocular pressure using Goldmann applanation tonometer without fluorescein. Eur J Ophthalmol. Jan-Feb;24(1):84-7. 5. Rathinam SR, Babu M. Algorithmic approach in the diagnosis of uveitis. Indian J Ophthalmol. 2013;61(6):255-62. 6. Lim AB, Park JH, Jung JH, et al. Characteristics of diffuse retinal nerve fiber layer defects in red-free photographs as observed in optical coherence tomography en face images. BMC Ophthalmol. 2020;20(1):16. 7. Suh MH, Kim DM, Kim YK, et al. Patterns of progression of localized retinal nerve fibre layer defect on red-free fundus photographs in normal-tension glaucoma. Eye (Lond). 2010;24(5):857-63. 8. Venkatesh P, Sharma R, Vashist N, et al. Detection of retinal lesions in diabetic retinopathy: comparative evaluation of 7-field digital color photography versus red-free photography. Int Ophthalmol. 2015;35(5):635-40. 9. Muftuoglu IK, Gaber R, Bartsch DU, et al. Comparison of conventional color fundus photography and multicolor imaging in choroidal or retinal lesions. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):643-9. |

