Ocular Surface HealthCheck out the other feature articles in this month's issue:- Lid Wiper Epitheliopathy: What the OD Needs to Know - How to Answer the “Why?” of Dry Eye - Artificial Tears: What Matters and Why - A Modern Approach to Meibomian Gland Dysfunction (earn 2 CE credits) |
Red eyes are one of the most common ocular complaints, whether occasional or chronic. After all, studies show that individuals with eye redness appear less happy, healthy and attractive than those with a whiter sclera.1 While the treatment for ocular redness should target the etiology of hyperemia, in the absence of obvious pathology clinicians can consider prescribing topical vasoconstrictors and supportive measures.
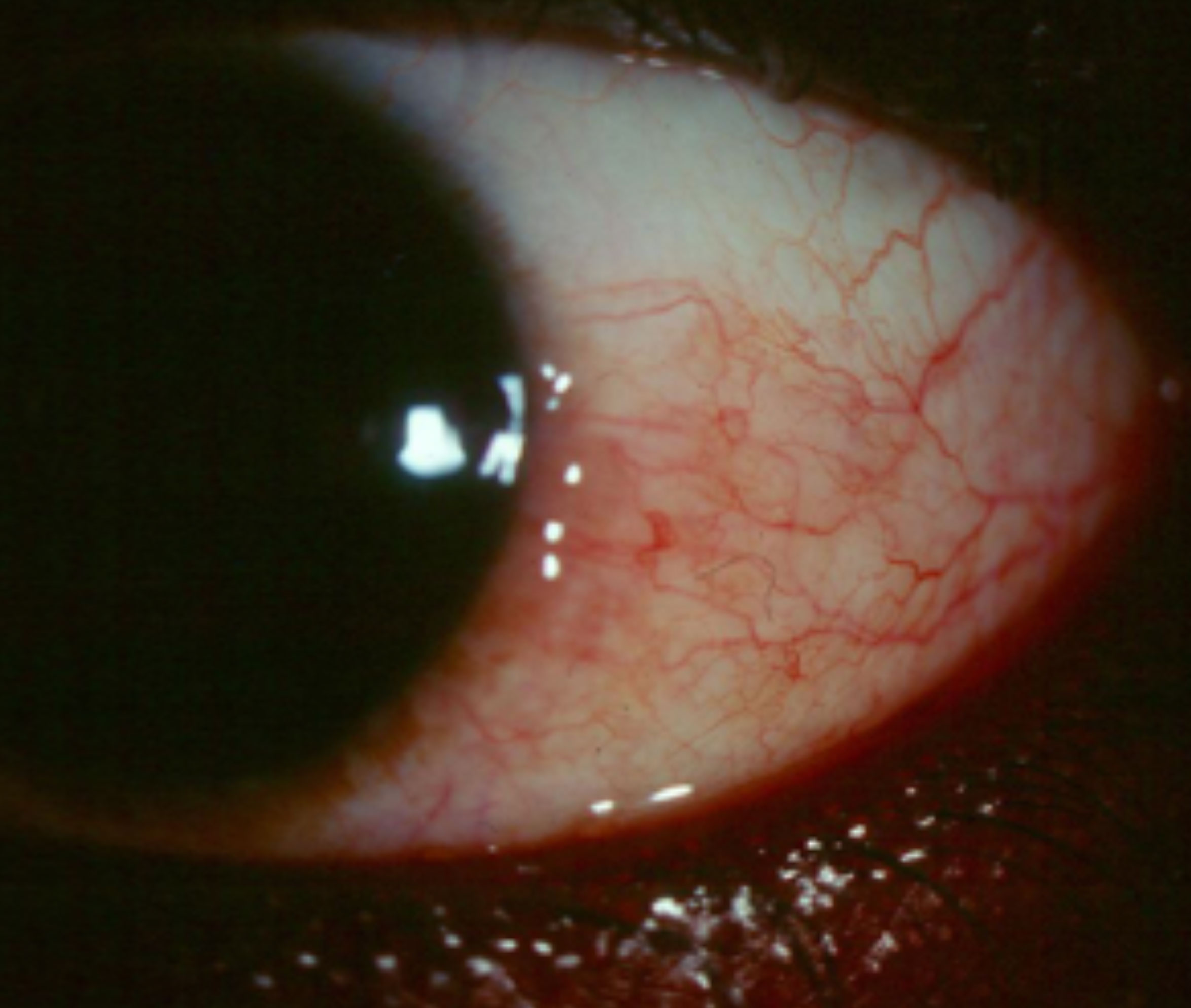 |
| Sectoral hyperemia such as this may be difficult to appreciate if the patient is using OTC Lumify. Click image to enlarge. |
Behind the Scenes
The conjunctiva and anterior episclera are nourished by blood vessels from the anterior and long posterior ciliary arteries, which stem from the ophthalmic artery. Redness, one of the five cardinal signs of inflammation, comes from vasodilation. The increased blood vessel diameters in response to tissue insult can lead to increased blood flow and leakage through the opened capillary sphincters. This results in edema, another sign of inflammation, increased inflammatory cells and mediators and, potentially, tissue damage.
In addition to tissue insult, an array of other factors, such as hypoxia and the presence of vasoactive amines, induce endothelial cell spreading, flattening and leakage through the capillary walls, causing vasodilation.
The quality of ocular redness is variable depending on the layer affected (conjunctiva, episclera, sclera or ciliary body) and the hue and pattern of hyperemia (circumlimbal, sectoral or diffuse), which may help differentiate between diagnoses and determine appropriate treatment options.
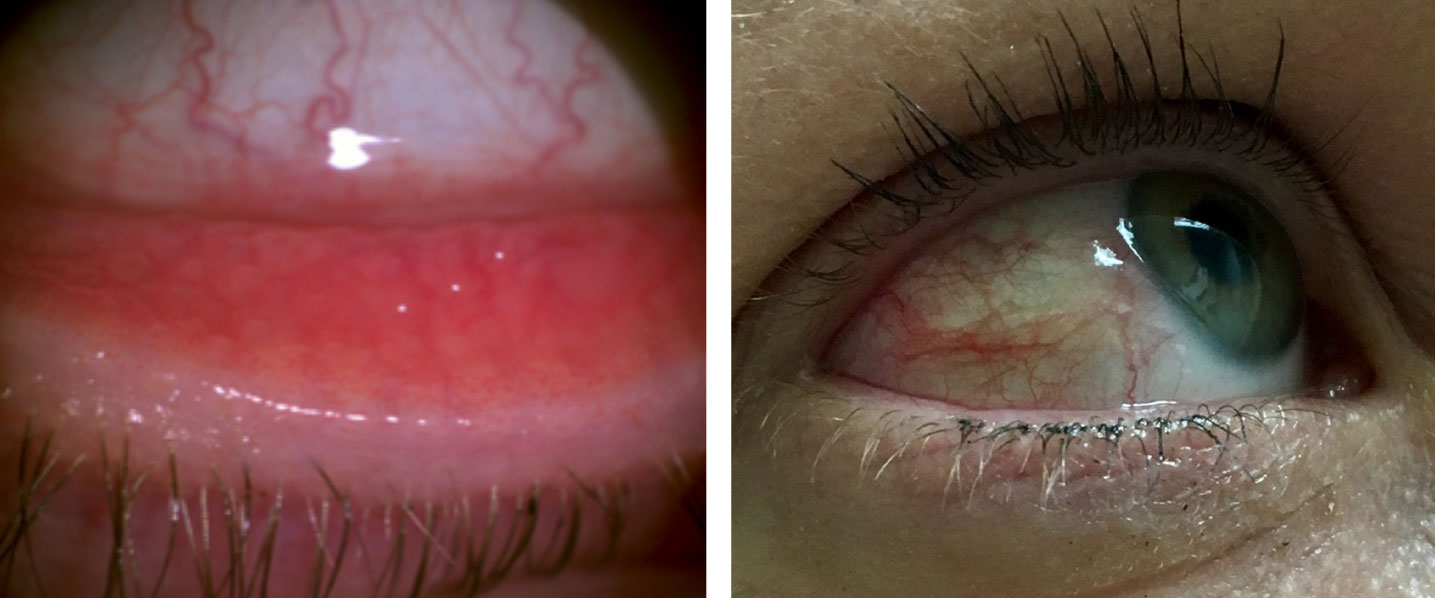 |
| Allergic conjunctivitis can present with bulbar and palpebral conjunctival hyperemia. Click image to enlarge. |
Contemporary Solutions
In the last three years, the FDA has approved a host of medications that can address red eyes, depending on the etiology:
Dry eye. In 2018, the FDA approved Cequa (0.09% cyclosporine A ophthalmic solution, Sun Pharma).2 This adds to the arsenal of prescription dry eye therapies that includes the well-established Restasis (0.05% cyclosporine A ophthalmic emulsion, Allergan) and Xiidra (5% lifitegrast ophthalmic solution, Novartis). A calcineurin inhibitor immunosuppressant, Cequa is indicated twice daily to increase tear production in adult patients with dry eye. The formulation uses nanomicelles to improve the penetration to ocular tissues.
Nanomicelles are ultramicroscopic structures made of exterior hydrophilic polar heads and an interior hydrophobic fatty chain.3 The outer layer allows the cyclosporine-carrying nanomicelle to move through the tear film and deliver the drug to the ocular surface.
While designed to treat dry eye, it can also reduce ocular surface irritation and redness.4,5 Ironically, the most common adverse reactions are pain and conjunctival hyperemia upon instillation; therefore Cequa is not recommended for immediate redness relief. Cequa is available in a box of 60 sterile, preservative-free, single-use vials.
 |
Eysuvis (loteprednol etabonate ophthalmic suspension 0.25%, Kala Pharmaceuticals) gained FDA approval in October 2020 for the short-term (two weeks) treatment of the signs and symptoms of dry eye. The Phase II and Phase III trials showed the treatment led to statistically significant improvements in conjunctival hyperemia and ocular discomfort severity. The company plans to launch the product by the end of 2020.6
Alcon’s OTC Systane Complete contains propylene glycol 0.6% and is preserved with Polyquad (polidronium chloride 0.001%).7 It is formulated with nano-droplet technology designed to disperse the active ingredient across the surface and stabilize the tear film. Propylene glycol can hold up to three times its own weight in water, increase the viscosity of solutions and form a protective layer over mucous membranes, which may lead to the reduction of ocular redness. Systane Complete is designed to provide relief for patients suffering from any type of dry eye. It is supplied in a sterile 10mL bottle.
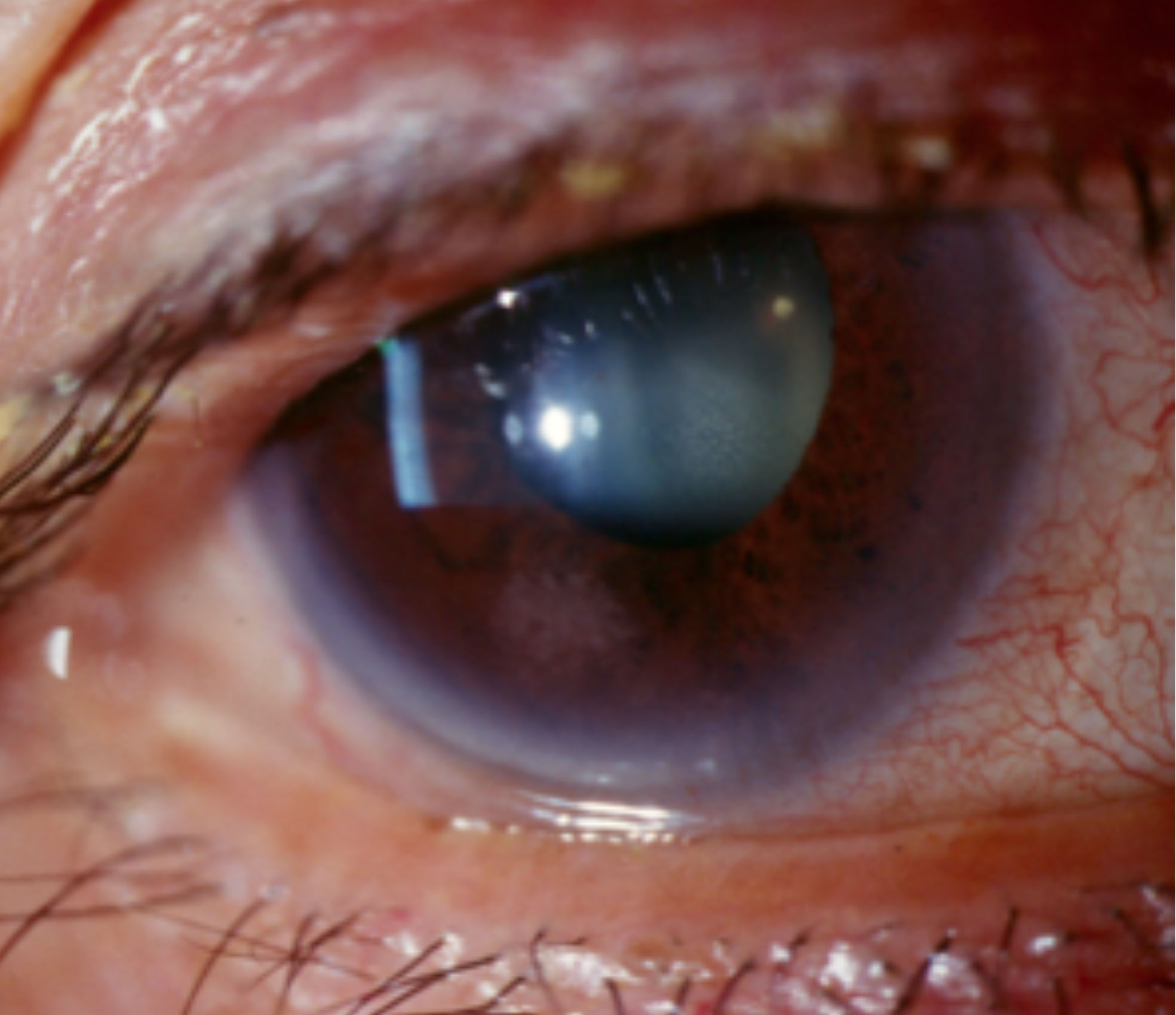 |
| Limbal hyperemia associated with anterior uveitis. Click image to enlarge. |
Refresh Relieva (Allergan), an OTC eye drop available since 2018, contains 0.5% carboxymethylcellulose, 0.9% glycerin and a glycerin-based solution called HydroCell.8 The drop may provide temporary relief of ocular irritation and burning, thereby decreasing some redness. Aqueous formulations of carboxymethylcellulose are known to reduce the production of inflammatory biomarkers.9 In addition, research shows formulations of carboxymethylcellulose with glycerin can relieve symptoms of dry eye in seven days and improve tear break-up time and corneal and conjunctival staining when used at least twice daily.10 The most common adverse side effect is temporary blurry vision. Refresh Relieva and Refresh Relieva PF are supplied in sterile 10mL bottles. Refresh Relieva for Contacts is supplied in a sterile 8mL bottle.
Allergic conjunctivitis. In 2017, the FDA approved Zerviate (0.24% cetirizine ophthalmic solution, Eyevance Pharmaceuticals) as a prescription BID drop for the treatment of ocular itching associated with allergic conjunctivitis in patients older than age two.11
Cetirizine, the active ingredient in Zyrtec (Johnson & Johnson), is a second-generation histamine-1 (H1) receptor antagonist with both antihistamine and mast-cell stabilizing properties, known to reduce ocular redness. Zerviate is formulated with glycerin and hydroxypropyl methylcellulose. The most commonly reported adverse reactions are ocular hyperemia, instillation site pain and reduced visual acuity.
While the goal of treatment may be to reduce redness due to the underlying allergic reaction, it is possible that some individuals will experience redness as a side effect of this medication. Zerviate is supplied in sterile 7.5mL and 10mL bottles and as a box of 30 sterile, preservative-free, single-use vials.
In February 2020, Pataday Once Daily Relief (0.2% olopatadine hydrochloride ophthalmic solution, Alcon) and Pataday Twice Daily Relief (0.1% olopatadine hydrochloride ophthalmic solution, Alcon) were FDA approved for OTC sale.12 Olopatadine is a well-established second-generation antihistamine and a mast-cell stabilizer.13 This dual-action selective H1 receptor antagonist inhibits histamine release from mast cells, preventing histamine-induced effects on conjunctival epithelial cells, including redness.
It is indicated for temporary relief of itchy eyes due to pollen, ragweed, grass, animal hair and dander in adults and children over the age of two. It has a low benzalkonium chloride (BAK) concentration of 0.01%, reducing potential side effects due to the preservative. The most common adverse reactions associated with olopatadine are blurred vision, punctate keratitis, dry eye, abnormal eye sensations and dysgeusia. Pataday Once Daily Relief is supplied in a box with two 2.5mL bottles. Pataday Twice Daily Relief is supplied in a 5mL bottle.
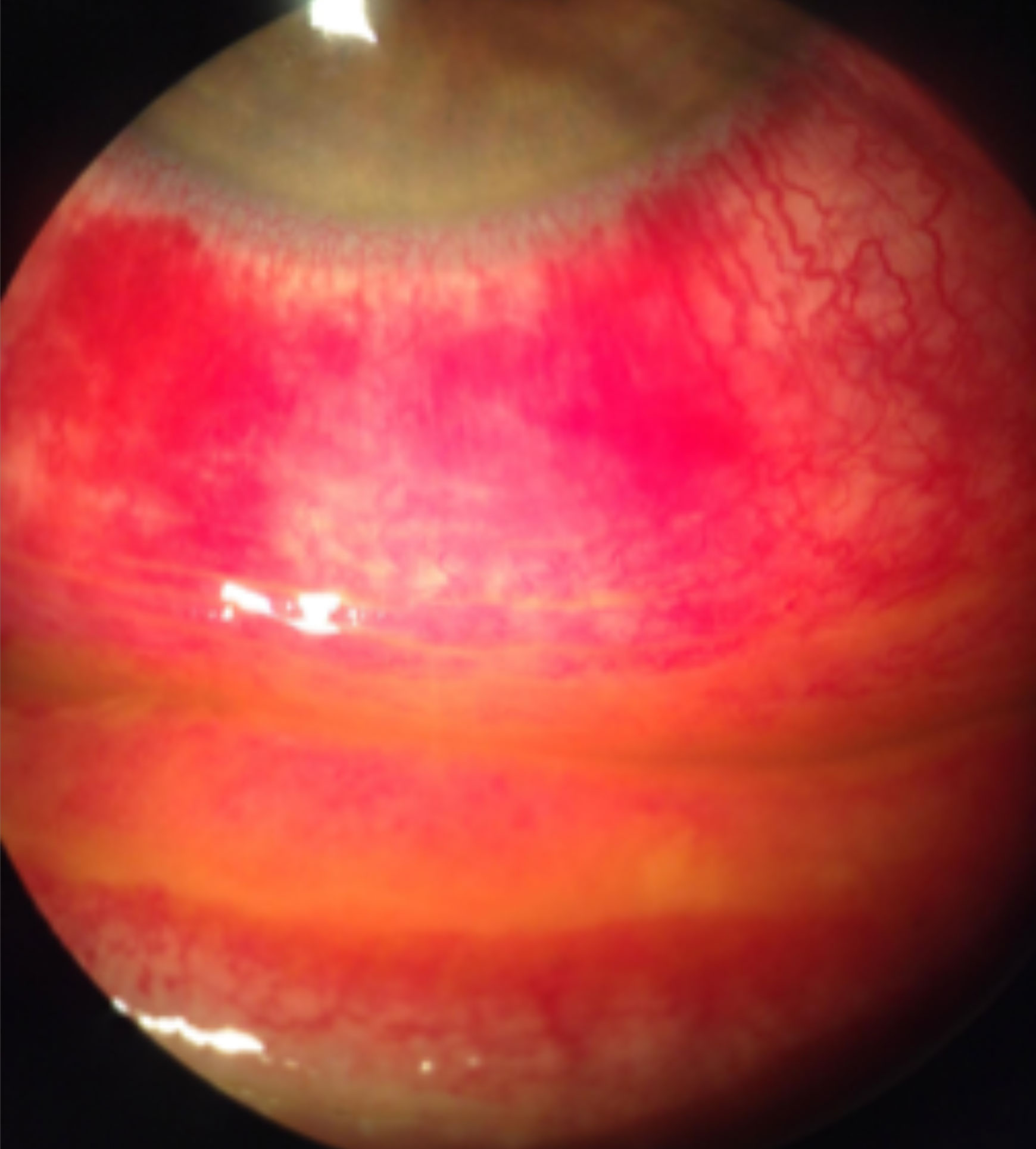 |
| Staining with fluorescein sodium reveals diffuse conjunctival hyperemia associated with a viral infection causing severe inflammation, subconjunctival hemorrhages and pseudomembranes. Click image to enlarge. |
Inflammation. Prescription corticosteroids are often used in the treatment of ocular inflammatory conditions, as they are highly effective in suppressing inflammatory, allergic and immune responses, including signs of redness. Corticosteroids suppress phospholipase A2 as well as the expression of cyclooxygenase/PGE isomerase (COX-1 and COX-2).
When a patient presents with hyperemia related to underlying inflammatory, allergic or immune responses, topical corticosteroids may help to rapidly improve signs and symptoms. However, they are known to delay healing and suppress the immune response and should, therefore, be judiciously prescribed—especially for keratitis of unknown etiology or for prolonged use, as steroids may exacerbate the prevalence of secondary bacterial, viral and fungal infections.14
Several options exist, including Pred Forte (1% prednisolone acetate, Allergan), Omnipred (1% prednisolone acetate, Novartis), dexamethasone, FML (0.1% fluorometholone acetate, Allergan), Flarex (0.1% fluorometholone acetate, Eyevance Pharmaceuticals) and Vexol (1% rimexolone, Novartis), all of which are ketone steroids that depend on liver metabolism to become inactive. These tried-and-true formulations are widely used as they are effective and readily available; however, caution is advised as their potential side effects include an increase in the intraocular pressure as well as posterior subcapsular cataract formation.14
Loteprednol, on the other hand, is an ester steroid that binds to glucocorticoid receptors and is rapidly metabolized into inactive metabolites. Loteprednol has consistently demonstrated a low propensity to increase intraocular pressure (IOP) in adults, even in known steroid responders.15 In addition, because of the ester group at C-20 position, loteprednol is unable to form covalent bonds with crystalline lens proteins, making it an unlikely culprit in cataract formation.16
Lotemax (loteprednol etabonate 0.5%, Bausch + Lomb), first approved in 2012, is available by prescription in three distinct formulations. As an ophthalmic suspension, it is indicated for the treatment of steroid-responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment. As a gel and ointment, it is indicated for the treatment of postoperative inflammation and pain following ocular surgery.
In February 2019, the FDA approved Lotemax SM (loteprednol etabonate 0.38% ophthalmic gel, Bausch + Lomb) with TID dosing for the treatment of postoperative inflammation and pain following ocular surgery.17 This new formulation takes advantage of submicron-size technology and uses particles that are significantly smaller than has been used in previous formulations; thus, the drug’s delivery to target tissues is improved.
Lotemax SM has a low BAK (0.003%) concentration and a neutral pH. The presence of glycerin, hypromellose and propylene glycol make it friendly to the ocular surface, and the use of polycarbophil prolongs its surface exposure. Safety and effectiveness have not been established in the pediatric population. Lotemax SM is available as 5g in a sterile 10mL bottle.
In August 2018, the FDA approved prescription Inveltys (1% loteprednol etabonate ophthalmic suspension, Kala Pharmaceuticals) as BID dosing to treat inflammation and pain following ocular surgery.18 This formulation takes advantage of mucus-penetrating particle drug delivery technology with nanoparticle size and a proprietary coating to prevent adherence of the drug to the tear film mucins, all of which is designed to improve drug delivery. It has a low BAK (0.01%) concentration. The safety and efficacy of this drop have not been established for children. Inveltys is available in a sterile 5mL bottle.
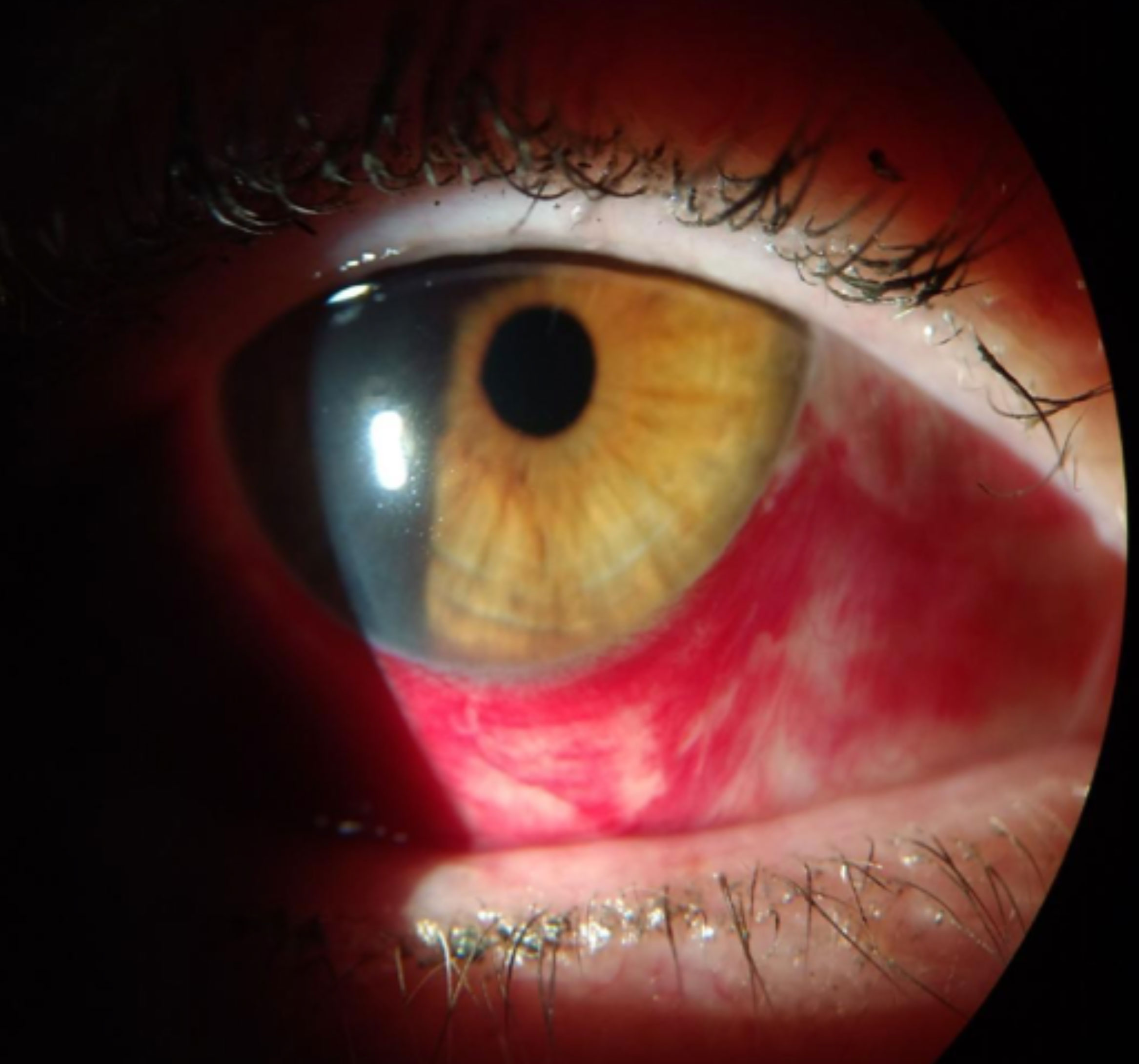 |
| Redness associated with a subconjunctival hemorrhage. Click image to enlarge. |
Vasodilation. In 2017, the FDA approved OTC Lumify (0.025% brimonidine tartrate ophthalmic solution, Bausch + Lomb) to relieve ocular redness due to minor eye irritation in patients older than age five.19 It can be instilled up to four times per day.
Currently available OTC vasoconstrictors are α-adrenergic receptor (AR) agonists that induce smooth muscle contraction but differ in their affinity for the α1- and α2- AR subtypes. Phenylephrine and tetrahydrozoline are considered selective α1-AR agonists while naphazoline and oxymetazoline are considered mixed α1/α2- AR agonists.20
Brimonidine is a third-generation selective α2-adrenergic receptor agonist. While α1-ARs appear to be present in arteries, α2-ARs appear to be primarily expressed in veins.19 Thus, the potential for vasoconstrictor-induced ischemia and responsive release of vasodilators is decreased with Lumify. Research suggests 0.025% brimonidine effectively reduces and maintains the reduction of ocular redness for four hours post-instillation without significant rebound following discontinuation. In addition, the pupils and IOP are not affected when used as directed. It has a low BAK (0.01%) concentration and is supplied in sterile 2.5mL and 7.5mL bottles.
Of interest, 0.2% brimonidine tartrate ophthalmic solution, indicated for lowering IOP in patients with open-angle glaucoma or ocular hypertension, can result in a rare but well-described late side-effect of acute anterior uveitis in some elderly patients.21,22 While the brimonidine concentration for IOP-lowering (0.1% to 0.2%) is considerably higher than for redness reduction as provided by Lumify—and the uveitis cases presented after many months of chronic use—clinicians should still be aware of this potential side effect, particularly since Lumify is available without a prescription.23
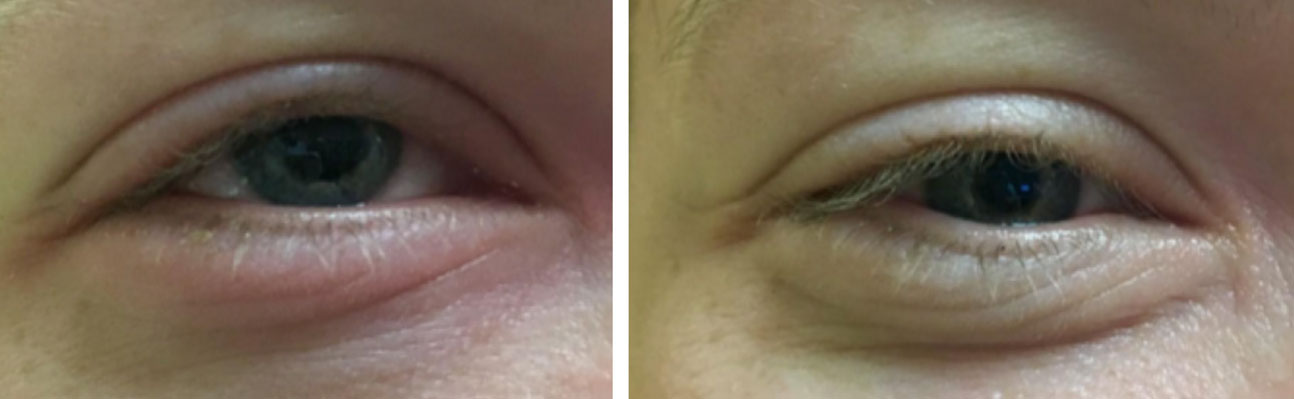 |
| Redness associated with a subconjunctival hemorrhage. Click image to enlarge. |
Treatment Pearls
The right medications to use when managing conjunctival hyperemia vary depending on the severity and nature of the underlying pathology. Primarily supportive medications, such as artificial tears, which can be kept in the fridge to be applied cold, can provide relief and decrease redness by limiting allergens and supporting the ocular surface, but their efficacy is limited in truly reducing redness. For some patients, cool compresses that induce vasoconstriction may be more beneficial.
Topical vasoconstrictors are more likely to be directly beneficial in reducing the severity of hyperemia, but they have limited efficacy and the formulations available prior to Lumify have a potential for tachyphylaxis or rebound effect.
Also, vasoconstrictors do not address the ocular redness trigger; medications that limit vascular dilation or perfusion will only address the blood and substances present within the vessels. Therefore, in the case of a subconjunctival hemorrhage, a vasoconstrictor may be beneficial initially to limit further bleeding, but a patient should be advised that the medication won’t eliminate the redness outside of the vessels associated with the bleed.
Patients often present with a complaint of redness and their primary concern is cosmetic in nature. However, the clinician must focus on addressing the underlying cause of the hyperemic reaction. Clinicians should specifically inquire during the case history about the presence of recent eye redness or the use of any agents designed to reduce ocular hyperemia. When patients report having prior redness, recent use of OTC agents or using old medications found at home, a careful slit-lamp examination is warranted. Clinicians should look for a pattern of any persistent redness and other potential signs of infection or inflammation, such as follicles or papillae, to reveal the diagnosis of an underlying condition. Here are a few patient examples to be on the lookout for:
- A patient does not have red eyes on gross examination due to the use of an OTC vasoconstricting agent but reports light sensitivity or foggy vision. This patient may require a more in-depth corneal and anterior chamber evaluation to rule out uveitis, even though one of the early signs associated with anterior uveitis—redness—is reduced or difficult to assess altogether.
- A patient suffering from adenoviral conjunctivitis may also turn to OTC redness reducing agents, leading to a delayed presentation for an ocular evaluation. This, in turn, could cause a missed or delayed diagnosis, furthering the transmission of the pathogen.
- A symptomatic contact lens wearer may self-treat with topical redness-reducing medications, masking the earliest symptom—hyperemia—of a poor contact lens fit or corneal hypoxia. The subsequent delayed care can have dire consequences for patients who are abusing their contact lenses, including a higher risk for infections and corneal disruption.
In the majority of ocular redness cases, supportive measures, including appropriate artificial tears and cool compresses, can provide some benefits. However, for many patients, addressing the underlying etiology of their ocular redness is key to truly providing long-lasting relief with minimal long-term sequelae.
Dr. Tyler is an associate professor at the Southern California College of Optometry at Marshall B. Ketchum University, Fullerton, CA.
Dr. Lewandowska is an assistant professor at the College of Optometry at Nova Southeastern University, Davie, FL.
| 1. Provine RR, Cabrera MO, Brocato NW, Krosnowski KA. When the whites of the eyes are red: A uniquely human cue. Ethology. 2011;117(5):395-99. 2. Sun Pharma Global FZE. Cequa [package insert]. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210913s000lbl.pdf. Revised August 2018. Accessed September 16, 2020. 3. Vadlapudi AD, Mitra AK. Nanomicelles: an emerging platform for drug delivery to the eye. Ther Deliv. 2013;4(1):1-3. 4. Prabhasawat P, Tesavibul N, Karnchanachetanee C, Kasemson S. Efficacy of cyclosporine 0.05% eye drops in Stevens Johnson syndrome with chronic dry eye. J Ocul Pharmacol Ther. 2013;29(3):372-7. 5. Deveci H, Kobak S. The efficacy of topical 0.05% cyclosporine A in patients with dry eye disease associated with Sjögren’s syndrome. Int Ophthalmol. 2014;34(5):1043-8. 6. Steroid Approved for Dry Eye. Rev Optom. October 27, 2020. www.reviewofoptometry.com/article/steroid-approved-for-dry-eye. Accessed November 6, 2020. 7. Alcon. Systane Complete lubricant eye drop. systane.myalcon.com/eye-care/systane/products/systane-complete. Accessed September 18, 2020. 8. Allergan. Refresh Relieva lubricant eye drops. www.refreshbrand.com/Practitioner. Accessed September 17, 2020. 9. Labetoulle M, Messmer EM, Pisella PJ, et al. Safety and efficacy of a hydroxypropyl guar/polyethylene glycol/propylene glycol-based lubricant eye-drop in patients with dry eye. BR J Ophthalmol. 2017;101(4):487-92. 10. Lievens C, Berdy G, Douglass D, et al. Evaluation of an enhanced viscosity artificial tear for moderate to severe dry eye disease: A multicenter, double-masked, randomized 30-day study. Cont Lens Ant Eye. 2019;42(4):443-49. 11. Eyevance Pharmaceuticals, LLC. Zerviate [package insert]. www.accessdata.fda.gov/drugsatfda_docs/label/2020/208694s006lbl.pdf. Revised February 2020. Accessed September 16, 2020. 12. FDA approves three drugs for nonprescription use through Rx-to-OTC switch process. U.S. Food and Drug Administration. www.fda.gov/news-events/press-announcements/fda-approves-three-drugs-nonprescription-use-through-rx-otc-switch-process. February 14, 2020. Accessed September 18, 2020. 13. Kam KW, Chen LJ, Wat N, et al. Topical olopatadine in the treatment of allergic conjunctivitis: a systematic review and meta-analysis. Ocul Immunol Inflamm. 2017;25(5):668-82. 14. David D, Berkowitz J. Ocular effects of topical and systemic corticosteroids. The Lancet. 1969;294(7612):149-51. 15. Sheppard JD, Comstock TL, Cavet ME. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Advance Thera. 2016;33(4):532-52. 16 Comstock TL, Sheppard JD. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opin Pharmacother. 2018;19(4):337-53. 17. Valeant Pharmaceuticals. Lotemax SM [package insert]. www.accessdata.fda.gov/drugsatfda_docs/label/2019/208219s000lbl.pdf. Accessed September 16, 2020. 18. Kala Pharmaceuticals. Inveltys [package insert]. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210565s000lbl.pdf. Revised August 2018. Accessed September 16, 2020. 19. Bausch & Lomb. Lumify redness reliever eye drops. www.lumifydrops.com/professional. Accessed September 17, 2020. 20. Torkildsen GL, Sanfilippo CM, DeCory HH, Gomes PJ. Evaluation of efficacy and safety of brimonidine tartrate ophthalmic solution, 0.025% for treatment of ocular redness. Curr Eye Research. 2018;43(1);43-51. 21. Byles DB, Frith P, Salmon JF. Anterior uveitis as a side effect of topical brimonidine. Am J Ophthalmol. 2000;130(3):287-91. 22. Beltz J, Zamir E. Brimonidine induced anterior uveitis. Ocul Immunol Inflamm. 2016;24(2):128-33. 23. Clemente-Tomás R, Arciniegas-Perasso CA, Hervás-Hernandis JM, et al. Hypertensive acute granulomatous anterior uveitis as a side effect of topical brimonidine. Arch Soc Esp Oftalmol. 2018;93(10):511-14. |

