Retinal Vascular Miniseries |
| Follow the links below to read the other installments in this miniseries: Part 1: Recognizing Abnormal Vasculature Part 3: Imaging Motion: a Review of OCT-A Part 4: Identify and Manage Retinal Vascular Tumors |
To help you better prepare to recognize vascular-based abnormalities, we provide here a guided tour of the blood vessels and structures surrounding the retina, as well as an explanation of the congenital anomalies associated with each structure. Though these aberrations are rare, they can have a significant impact on vision and treatment of visual complications. They can also serve as a marker for underlying systemic illnesses and dictate the clinician’s work-up and treatment plan.
This feature is the second in a four-part series that offers an in-depth look at the intricacies of retinal vasculature to highlight the importance of the vascular supply in maintaining retinal integrity and function.
Retinal Blood Supply and Drainage
The ophthalmic artery (OA), the first major subdivision of the internal carotid artery on each side, is the first in a series of several arteries responsible for nourishing various ocular structures and adnexa.1-3 The retina is supplied with blood through two branches of the OA: the central retinal artery (CRA) and the posterior ciliary arteries.1-3 The first branch, the central retinal artery, runs along the optic nerve, passing through the lamina cribrosa and entering the optic disc nasal to the postocular center.1 It then branches superiorly and inferiorly, dividing further into nasal and temporal arcades (branches). These branches continue to bifurcate and supply blood to the inner retinal layers.1,2 The CRA is a terminal branch of the OA and serves as the primary source of blood to the retina.2,3The outer and middle retina is nourished by the choroid, which gets its blood supply from a different branch of the OA called the posterior ciliary arteries.3,4 These arteries vary in number per individual, ranging from one to five, and divide further into several short arteries that supply the proximal choroid and optic nerve head. They pierce the sclera and continue as long ciliary arteries to supply the distal choroid.3
Venous drainage of the inner retina occurs first through the branch retinal veins into the central retinal vein, which emerges from the meningeal sheath of the optic nerve. The vortex veins of the choroid are responsible for draining the outer retina and choroid. They drain into the superior and inferior ophthalmic veins. These, and the central retinal vein, flow into the cavernous sinuses in the middle cranial fossae.1 Blood supplied by this route enters the systemic circulatory system via the petrosal venous and sigmoid sinuses, which terminate in the internal jugular veins in the posterior cranial fossae.
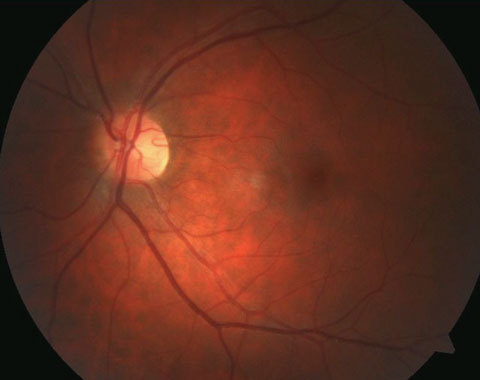 |
| Pictured here is a cilioretinal artery coming off the temporal rim. Click image to enlarge. |
Cilioretinal Arteries
There are anatomic variations in which an arterial branch originating from the posterior ciliary arteries or choroid, or both, assists the CRA in supplying the inner retinal layers.3,5 Studies show it is the most common congenital vascular anomaly of the retina, found in 6% to 32% of individuals.3,6,7 A study using fundus photography and fluorescein angiography (FA) to aid in observation of cilioretinal arteries estimated the prevalence at 32.1% of eyes, suggesting these entities may have been underdiagnosed without the use of ancillary testing in earlier studies.8 Uncommonly, multiple cilioretinal arteries can be present in one eye.8 The incidence of bilateral presentations is approximately 14% to 18%.3 Studies suggest genetics play a strong role in cilioretinal artery development.5Cilioretinal arteries appear clinically as vessels that originate at the disc margin and arch outwards. The sharp loop caused by the arch is an important diagnostic feature, as is the absence of a direct connection to CRA.8 They may be singular, multiple and vary in size from minor to substantial. On FA, cilioretinal arteries fill seconds before the rest of the retinal circulation because they gain their blood supply from the choroid.9
These vessels commonly supply the fovea, followed by the lower temporal region of the retina; occasionally they supply regions directly temporal to the disc.8 Only a few cases have been reported in a nasal location.5,8 Even more rare are cases in which a cilioretinal artery supplies the entire retina, suggesting that they may create an anastomotic network with normal CRA branches.3
A cilioretinal artery may be beneficial in cases of a central retinal artery occlusion. Here, the presence of this congenital anomaly can prevent catastrophic vision loss by providing an alternate blood supply to the fovea and maintaining circulation in the event of a CRA occlusion.3,5,9 In a similar manner, it can also aid in retaining central vision in cases of advanced primary open angle glaucoma (POAG), due to the increased level of circulation to the temporal rim of the optic nerve head.10 However, it has not been shown to markedly influence the pattern of neuroretinal rim loss or parapillary atrophy progression in POAG.11
While the presence of a cilioretinal artery can be of great clinical benefit, it may also pose potential risk, such as in instances of cilioretinal artery occlusion. However, unlike in cases of central or branch artery obstructions, visual prognosis is often good, with 20/40 visual acuity or better in more than 90% of cases.9,12
In addition to the risk of occlusion, the cilioretinal artery is also associated with an increase in blood flow velocity. This can boost the incidence of diabetic macular edema.13 One case in the literature reported reduced visual acuity and mild amblyopia due to an aberrant branching cilioretinal artery and macular thickening secondary to its compromise.6 Finally, patients with this anomaly can fall victim to pathological processes such as macroaneurysm, although this is rare.14
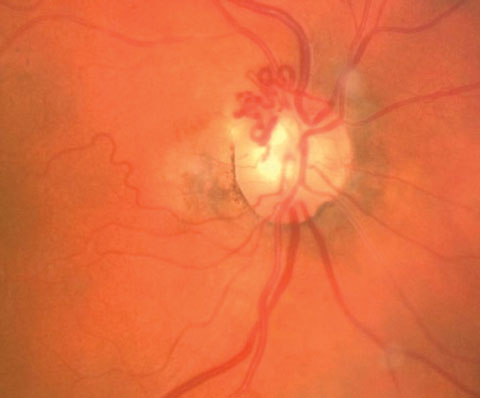 |
| Pictured here are collateral vessels. |
The Collateral Vessel
Retinal collateral vessels (CVs) originate from the existing retinal capillary network and become evident when one vessel is occluded, leaving the adjacent vascular channels operative.15,16 Collateral vessels arise from processes that try to correct or compensate for vessel obstruction. Here, the obstructed vessel joins with an unobstructed one.16 Collateral vessels have similar characteristics as normal retinal vessels; the channel they form connects inner retinal layers.16 Although there is potential for CVs to be preexisting, they have no clinical significance and typically remain stationary throughout life in healthy patients.17CVs are generally classified into three categories: arterioarteriola, arteriovenous collaterals and venovenular collateral.
Arterioarteriola collaterals become evident following a branch retinal artery obstruction and, rarely, in central retinal artery occlusion.15,16 This subtype is seen infrequently and appears within weeks of arterial occlusion. The timing of their manifestation is not early enough to forestall the damage from the occlusive event, but serves instead as a marker of previous arterial occlusive disease on funduscopic examination.15
Arteriovenous collaterals may arise and fill in cases of capillary bed obstruction to allow blood to cross from the arterial side to the venous side of retinal circulation. Examples of retinal pathology that promote this include conditions such as diabetic retinopathy, long-standing glaucoma, sickle cell retinopathy and Leber’s multiple miliary aneurysms.15,16
A venovenular collateral, the most frequently encountered subtype of collateral vessels, manifests following venous obstruction to provide an alternative means of blood flow from congested retinal areas relieving the “log jam” at the site of the thrombosis and mitigating the secondary ischemia produced by the poor perfusion induced by the venous stasis, preserving retinal function.16,18 They become patent as a result of the pressure alteration produced by a vein occlusion and resultant increase of the intraluminal blood flow within the retinal vessels. This mechanism leads to an increase in the volume of blood that flows through the CVs.17
Investigators presume that any capillary channel can be formed into a larger-caliber vessel, such as an arteriole or vein, if circumstances initiate the process.15 In the case of a branch vein occlusion (BRVO), CVs arise in approximately 22% of cases, allowing venous drainage into alternative, adjacent areas of the retina; these manifestations are associated with superior functional improvements and better visual prognosis.17,18 Less is known regarding the benefit of CVs in central vein occlusion (CRVO). Some studies report an association with improved visual prognosis, while others hypothesize that their presence may delay the resolution of macular edema, hence worsening the prognosis.16,19
CVs are observed as early as weeks after venous obstruction.15 They initially appear as small, tortuous vessels crossing the horizontal raphe to bypass the acquired venous blockage.15,16 They take on a similar tortuous appearance in a CRVO, presiding over the optic disc.16,19 Over a course of approximately six to 24 months, CVs mature and stabilize, appearing less tortuous.18
It is sometimes difficult to distinguish CVs from neovascularization on ophthalmoscopic evaluation. The predominant difference between these two vessel types lies in their appearance: CVs appear as normal, tortuous vessels that can be traced from vein to vein, artery to artery, artery to vein or vein to artery. Neovascular vessels are small and resemble “angel hair pasta.” They are extremely tortuous and are nowhere close to normal-caliber vessels. Furthermore, their behavior on fluorescein angiography (FA) demonstrates leakage, where neovascular membranes leak but CVs typically do not.18
Congenital Retinal Macrovessel
An aberrant blood vessel (either an artery or vein) that courses over the macula with its tributaries crossing the horizontal raphe, to either supply or drain the macular region, is known as a congenital retinal macrovessel (CRM).20-23 These are rare, reported to only occur in approximately one in 200,000 cases.21,24 CRMs are typically unilateral and are thought to develop from anomalous embryonic tissues during the 15th and 16th weeks of gestation, at which time the differentiation of the mesenchymal cells invade the retinal nerve fiber layer.20,21,25 It is often categorized under group one of the retinal arteriovenous anastomoses classification system, which describes single or multiple vessels with direct arteriovenous communication and interposition of a capillary network.22CRMs are diagnosed on fundus examination and can be confirmed with FA. Typical FA findings show early filling of the vein, followed by a slight delay in emptying. An atypical capillary network is also revealed through FA.24 Optical coherence tomography (OCT) can be a useful tool in the diagnosis of CRM, as it may exhibit a disruption within the architecture of the foveal avascular zone.26
The presence of these isolated vascular malformations is benign and often an incidental finding on routine retinal examination.25 Rarely, CRMs can cause a decrease in vision precipitated by the development of macular hemorrhage, foveal cysts, serous macular detachment, or simply the presence of the vessel coursing over the macula.21,22 Arterial CRM, in conjunction with leaking retinal arterial macroaneurysm, can cause a decrease in vision due to macular impingement, according to investigators.23 Some evidence suggests an association of CRMs with systemic vascular formations, as is seen in arteriovenous malformation (Wyburn-Mason syndrome), but this has only been recorded in one case revealing a coincident ipsilateral venous malformation within the left frontal lobe, suggesting that not all of these patients would need emergent neuro-imaging.25
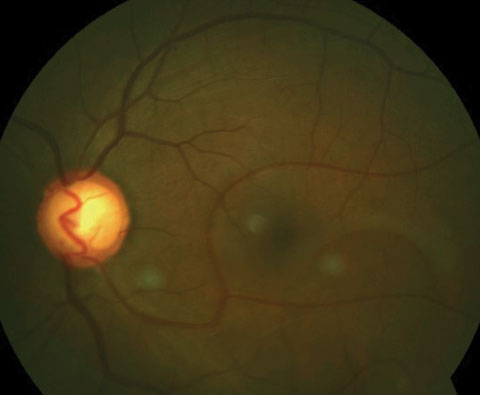 |
| Here is an inferior congenital retinal macrovessel extending over the horizontal raphe. Click image to enlarge. |
Retinal Arteriovenous Malformation
Also known as racemose hemangioma and retinal arteriovenous communication (angiomatosis retinae), retinal arteriovenous malformation (AVM) is a rare, congenital but nonhereditary sporadic phacomatosis arising from the same tissue normal to the area.27,28 It is characterized by marked dilation of venous and arterial tissue.29The condition presents unilaterally and may either be confined to a specific area of the retina or diffusely widespread.29,30 Most commonly, retinal AVMs are found in the superior temporal quadrant, followed by the papillomacular bundle and least commonly nasal to the optic disc.29 On FA, retinal AVM fill rapidly and do not leak.29 On OCT, retinal AVMs appear as prominent, intraretinal vessels that correspond in area to the abnormal retinal vasculature on fundus examination. Though they vary in depth, these vessels typically do not penetrate deeper than the outer nuclear layer of the retina.30
Retinal AVMs are classified into three subtypes. Type 1 involves a capillary plexus or network connecting artery and vein. Type 2 retinal AVMs do not involve this adjoining capillary network, and are classified based upon their direct arterial and venous communication. Finally, the third type is characterized by intertwined and convoluted connections that it make it difficult to differentiate between the arterial and venous components.29,30 Type 3 is also often associated with concurrent lesions of the optic nerve, chiasm and the cerebral cortex, as in Wyburn-Mason syndrome.29
Typically, retinal AVMs are nonprogressive and not visually threatening.29,31 Occasional, rare ocular complications that lead to visual decline can arise from these high-flow developmental vascular anomalies.27,29 The most common cause of visual disruption is venous occlusion, which is thought to arise from the high, turbulent venous blood flow and decreased arterial pressure leading to thrombus formation and surrounding areas of induced retinal ischemia.32
Vitreous hemorrhage may occur as a consequence of the same process. Increases in hydrostatic pressure on the venous side of the malformation can also produce retinal and vitreous leakage.30,33 Neovascular glaucoma has been documented in cases of retinal AVMs occurring secondary to retinal ischemia.27,28 Macular edema is also possible.34 Associated ocular conditions include morning glory syndrome, macular holes, Sturge-Weber disease, Vogt-Koyanagi-Harada disease, Duane’s retraction type 1 and retinal macroaneurysms.27
Wyburn-Mason syndrome, also known as Bonnet-Dechaume-Blanc syndrome, occurs in approximately 30% of retinal AVMs and is accompanied by angiomas of the brain, most commonly the midbrain, or within the face.27,35 Wyburn-Mason syndrome results from a disruption in embryological development within the vascular mesoderm.28 Associated intracranial AVMs can result in neurological morbidity and even death, making retinal AVMs of great clinical importance when diagnosed.28 The presence of these intracranial or orbitocranial lesions can also result in optic atrophy and resultant vision loss.27,28
Though the risk of ocular complication and vision loss is rare, retinal AVMs require prompt referral to neurology or neuro-ophthalmology departments for neuroimaging to rule out the possibility of associated intracranial or facial AVMs. Prompt magnetic resonance imaging and cerebral angiography can identify these conditions, at which point referral for a neurosurgical consult is recommended.28 Due to advancements in surgical and radiation techniques, the intracranial AVMs can sometimes be fully eliminated without compromising cerebral blood flow.28
Although these congenital anomalies are not often encountered, they carry great clinical significance. Some of these conditions can appear funduscopically in response to a vascular event, while others may point to underlying systemic abnormalities. Despite the rarity of ocular complications in these cases, prompt identification and appropriate referral or management can aid in the prevention of visual decline.
Dr. Labib is an assistant professor with the Eye Institute at Salus University.
Dr. Gurwood is a clinical director and professor with the Eye Institute at Salus University.
Dr. Meagher is a primary care optometrist with the Eye Institute at Salus University.
|
1. Remington LA. Retina. Clinical Anatomy and Physiology of the Visual System. 3rd ed. St. Louis, MO: Elsevier/Butterworth Heinemann;2012:87-8. 2. Kiel JW. The Ocular Circulation. San Rafael, CA: Morgan & Claypool;2011. Web. 3. Michalinos A, Zogana S, Kotsiomitis E, et al. Anatomy of the ophthalmic artery: a review concerning its modern surgical and clinical applications. Anat Res Int. 2015:591-6. 4. Anand-Apte B, Hollyfield JG. Developmental anatomy of the retinal and choroidal vasculature. Elsevier Ltd. 2010;9-15. 5. Tarnhaj NC, Munich IC, Kyvik KO, et al. Heritability of cilioretinal arteries: a twin study. Invest Ophthalmol Vis Sci. 2005;46(10):3850-4. 6. Bonyadi MH. Amblyopia associated with prominent cilioretinal artery. J Opthalmic Vis Res. 2014;9(4):520-1. 7. Nuo X, Cui Y, Zhonghai G. Bilateral congenital venous tortuosity and dilatation combined with cilioretinal artery: a photographic essay. Inter Med Case Rep J. 2016;9:91-3. 8. Jain IS, Nagpal KC. Vessels at the disc margin (cilioretinal and other stimulating cilioretinal vessels). Ind J Opthalmol. 1972;20(4):141-4. 9. Yanoff Y, Duker JS. Retina: Ophthalmology. 4th ed. St. Louis, MO: Mosby; 2004:544-5. Print. 10. Lee SS, Schwartz B. Role of the temporal cilioretinal artery in retaining central visual field in open-angle glaucoma. Ophthalmology. 1992;99(5):696-9. 11. Budde WM, Jonas JB. Influence of cilioretinal arteries on neuroretinal rim and parapapillary atrophy in glaucoma. Invest Ophthalmol Vis Sci. 2003;44(1):170-4. 12. Stoffelns BM, Laspas P. Cilioretinal artery occlusion. Klin Monbl Augenheilkd. 2015;232(4):519-24. 13. Landa G, Amde W, Haileselassie Y, et al. Cilioretinalarter ies in diabetic eyes are associated with increased retinal blood flow velocity and occurrence of diabetic macular edema. Retina. 2011;31(2):304-11. 14. Giuffrè G, Montalto FP, Amodei G. Development of an isolated retinal macroaneurysm of the cilioretinal artery. Br J Ophthalmol. 1987;71(6):445-8. 15. Henkind P, Wise GN. Retinal neovascularization, collaterals, and vascular shunts. Brit J Ophthal. 1974:413-22. 16. Landa G, Rosen RB. New patterns of retinal collateral circulation are exposed by a retinal functional imager (RFI). Br J Opthalmol. 2010;94(7):54-8. 17. Kokolaki AE, Georgalas I, Koutsandrea C, et al. Comparative analysis of the development of collateral vessels in macular edema due to branch retinal vein occlusion following grid laser or ranibizumab treatment. Clin Ophthalmol. 2015;9:1519-22. 18. Im, CY, Lee SY, Kwon OW. Collaterals in branch retinal vein occlusion. Korean J Ophthalmol. 2002;16:82-7. 19. Santiago JG, Walia S, Sun JK. Influence of diabetes and diabetes type on anatomic and visual outcomes following central retinal vein occlusion. Eye. 2014;28:259-68. 20. Makino S, Endoh K, Tampo H. Retinal microvascular abnormalities in neurofibromatosis Type 1 associated with congenital retinal macrovessels. Case Reports in Ophthalmol Medi. 2013;5:1-3. 21. Ascaso FJ. Spontaneous resolution of central serous chorioretinopathy in a patient with congenital retinal macrovessel. Images Cardiovasc Med. 2011;124:904-5. 22. Geol N, Kumar V, Seth A, et al. Branch retinal artery occlusion associated with congenital retinal macrovessel. Oman J Ophthalmol. 2014;7(2):96-7. 23. Goel N, Kumar V, Seth A, et al. Intravitreal bevacizumab in congenital retinal macrovessel with retinal arteriolar macroaneurysm. Saudi J Ophthalmol. 2015;29(11):292-4. 24. de Crecchio G, Pacente L, Alfieri MC, et al. Congenital retinal macrovessels: A “low visual acuity” case report with a 14-year follow up. Acta Ophthalmologica Scandinavica. 1999;77:474-5. 25. Sanfilippo CJ, Sarraf D. Congenital macrovessel associated with cystoid macular edema and an ipsilateral intracranial venous malformation. Ret Cases Brief Rep. 2015;9:357-9. 26. Perez-Carro G, Miranda-Rollon M, Jucenda-Moreno C, et al. Congenital retinal macrovessels: A discovery by chance. Arch Soc Esp Ophthal. 2008;83(4):273-6. 27. Qin X, Huang C, Lai K. Retinal vein occlusion in retinal racemose hemangioma: a case report and literature review of ocular complications in this rare retinal vascular disorder. BMC Ophthalmol. 2014;5(10):101-4. 28. Reck SD, Zacks DN, Eibschitz-Tsimhoni M. Retinal and intracranial arteriovenous malformations: Wyburn-Mason syndrome. J Neuroophthalmol. 2005;25(3):205-8. 29. Liu W, Sharma S. Abnormal Retinal Vessel. Can Fam Physcian. 2005;51(2):203–9. 30. Winter E, Elsas T, Austeng D. Anti-VEGF treating macular oedema caused by retinal arteriovenous malformation – a case report. Acta Ophthalmol. 2014;92:192-3. 31. Ehrt O. Slowly progressive retinal arteriovenous malformation and relative amblyopia. Arch Ophthalmol. 2004;122(3):408-9. 32. Mansour AM, Walsh JB, Henkind P. Arteriovenous anastomoses of the retina. Ophthalmol. 1987;94(1):35-40. 33. Mansour AM, Wells CG, Jampol LM, et al. Ocular complications of arteriovenous communications of the retina. Arch Ophthalmol. 1989;107(2):232-6. 34. Soliman W, Haamann P, Larsen M. Exudation, response to photocoagulation and spontaneous remission in a case of bilateral racemose haemangioma. Acta Ophthalmol Scand. 2006;84(3):429-31. 35. Théron J, Newton TH, Hoyt WF. Unilateral retinocephalic vascular malformations. Neurorad. 1974;7(4):185-96.1. |

