 |
|
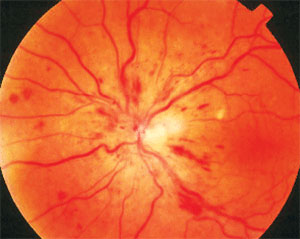
The group of patients receiving ranibizumab injections fared better in many areas than those receiving sham injections. Photo: Joseph W. Sowka, OD, and Alan G. Kabat, OD. Click image to enlarge. |
The phase III Ranibizumab for the Treatment of Macular Edema after Central Retinal Vein Occlusion Study: Evaluation of Efficacy and Safety (CRUISE) showed that central retinal vein occlusion (CRVO) patients treated with ranibizumab were found to have improved visual acuity and macular edema at the six-month endpoint. Further subanalysis of this dataset has also revealed improved macular nonperfusion in patients treated with ranibizumab.
Researchers wanted to identify additional anatomic and clinical endpoints that could signify beneficial disease modification following anti-VEGF therapy, so they reviewed available CRUISE study data and determined the beneficial disease-modifying effects through a reduction in retinal hemorrhage, neovascularization and papillary swelling. Exploratory analysis also found that ranibizumab treatment resulted in a decrease in fluorescein angiogram leakage area. These differences were seen as early as three months.
The study performed a post-hoc analysis of a total of 392 patients with macular edema after CRVO who were randomized 1:1:1 to receive monthly intraocular injections of either 0.3mg or 0.5mg of ranibizumab or sham injections. At six months, there was a reduction in the ranibizumab groups compared with sham groups with respect to total area of retinal hemorrhage (median change from baseline in disc areas: -1.17 [sham], -2.37 [ranibizumab 0.3mg], -1.64 [ranibizumab 0.5mg]), development of disc neovascularization (prevalence: 3% [sham], 0% [ranibizumab 0.3mg], 0% [ranibizumab 0.5mg]) and presence of papillary swelling (prevalence: 22.9% [sham], 8.0% [ranibizumab 0.3mg], 8.3% [ranibizumab 0.5mg]). The team noted no difference between groups in collateral vessel formation.
Blocking VEGF in patients with diabetic retinopathy and vein occlusion reduces vascular permeability, which may help reduce retinal hemorrhage. Therefore, ranibizumab and other anti-VEGF agents may allow for a more rapid resolution to normal retinal circulation than if left to natural history alone.
“Assessing area of retinal hemorrhage rather than counting the number of hemorrhages is a better reflection of how much of the fundus is affected by this feature of retinal vein occlusion,” the researchers noted. “This provides a more accurate quantitative measurement that may be useful for the development of a future severity scale.”
“Ultimately, the evaluation, classification and quantification of these anatomic endpoints could potentially be used for determining prognosis and a severity scale (much like that used for diabetic retinopathy) for eyes with CRVO,” they went on to conclude.
Huang JS, Khurana RN, Ghanekar A, et al. Disease-modifying effects of ranibizumab for central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. October 6, 2021. [Epub ahead of print]. |

