Annual Innovations in Eye Care IssueFollow the links below to read other articles from our annual Innovations in Eye Care issue: Breaking the Burden: A New Way to Deliver Anti-VEGF AI for DR: “The Digital Doctor Will See You Now” |
I had the pleasure of seeing the most delightful patient recently. A gentleman in his mid 70s, he was presenting for a follow-up visit for our current clinical trial of recombinant human nerve growth factor (rhNGF) eye drops at Byers Eye Institute at Stanford. The goal of this medication is to preserve the life of retinal ganglion cells (RGCs) and slow the progression of visual field (VF) loss. As I reviewed his VFs and data from the prior months, my heart sank. He had devastatingly advanced, end-stage glaucoma. His VFs were dark, except for the smallest central island. When we spoke, he began asking me several questions that we researchers ask ourselves—“Why does my disease progress even though I’ve never had a pressure greater than 12mm Hg? Are other factors at play aside from the eye pressure? Are we going to find a way to treat people like me?”
Finding those answers is specifically what my research team is working toward as we investigate neuroprotection and vision restoration for glaucoma.
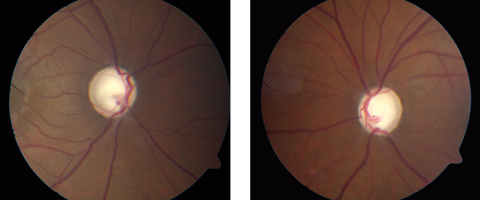 |
| If promising new research is on the right track, patients may be able to avoid this kind of glaucomatous optic nerve damage in the future. Click images to enlarge. |
Reviving Ganglion Cells
This is an exciting time to be part of glaucoma research. We have 60 patients enrolled in an eight-month, double-masked, randomized Phase Ib clinical trial testing rhNGF, including treatment and placebo controlled arms (clinicaltrials.gov, #NCT02855450). The patients included have clinical evidence of progressive glaucomatous RGC dysfunction based on VF and structural imaging modalities. The trial requires residual VF preservation in at least one quadrant—i.e., something left to save. Exclusion criteria include vision loss from other causes and recent intraocular surgery.
The design is atypical for glaucoma trials in that the primary outcome is not measured in intraocular pressure (IOP) but rather visual function. We are measuring functional endpoints including visual acuity, VF and electrophysiology, and structural endpoints including RGC layer thickness by optical coherence tomography. Translating laboratory-based research into clinical-based research, we are working with glaucoma patients at all stages. Many see this trial as their last line of hope, although other similarly designed trials investigating other therapies are also in progress at Stanford and a few other centers. These trials are testing whether candidate treatments could revive sick, poorly functioning RGCs and enable them to avoid ultimate death, but on a non-IOP mediated basis. What we are learning will hopefully be applied to future patients at earlier stages of their disease, leading to more preservation of functional vision.
The ability to maneuver in an environment without bumping into objects, to drive a car, to read printed material are all activities that many of us take for granted. The aim of these studies is to find new therapies that could halt glaucoma before it becomes debilitating.
Under Pressure
As we know, glaucoma is a neurodegenerative disease that causes an optic neuropathy, mostly affecting RGCs and leading to visual field defects. It is irreversible and can be devastating. Estimates predict glaucoma patients worldwide in 2020 will reach 79.6 million, with 5.9 million people estimated to be bilaterally blind from primary open-angle glaucoma (POAG).1
Despite diligent research, we simply don’t understand much about the pathophysiology of glaucoma. We have evidence that IOP is an important risk factor but, perhaps more importantly, we now realize that it is merely one factor.2 As with my patient, some with the disease maintain low pressures yet continue to progress. Through ocular hypotensive therapy, surgical or laser intervention, or both, these patients maintain a low target pressure range; however, their glaucoma continues to advance and in some cases ultimately leads to loss of functional vision or blindness. Questions physicians should be asking include ‘how much do vascular and blood flow factors affect the glaucomatous damage of the optic nerve and how can we use those factors for treatment?’ ‘Are we dealing with distinct pathologic processes that result in diverse trajectories and how can we identify the patients in each group?’ ‘Are there new therapeutic avenues we should pursue, such as neurotrophic factors, to prevent RGC death?’
As we have realized the limitations of IOP lowering in glaucoma, neuroprotection has emerged as a critical area of research. In glaucoma, neuroprotection has been defined as an intervention independent of IOP reduction that prevents RGC death.3 As a 2012 investigation shows, the ideal time to protect neurons is before extensive damage is done.4 We unfortunately don’t catch all patients at that early stage, but intervention must at least be before near complete or complete RGC death. Though the balance of the discussion will relate to therapies for neuroprotection, early detection and prevention are essential components of glaucoma care.3,4
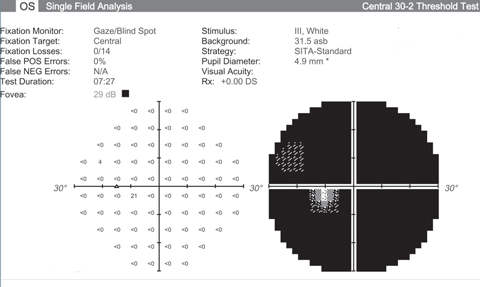
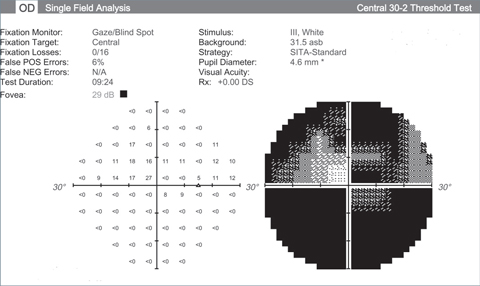 |
| Visual fields like these from an end stage glaucoma patient show the lack of function we’re hoping neuroprotective advancements can halt. Click images to enlarge. |
 |
Potential Pharmaceuticals
Thanks to the persistent work of many scientists around the world, we have drug targets and candidate therapies that inspire hope for better patient outcomes. Though much of this research is still taking place in the basic science labs, it is moving into the clinical realm. These are some of the agents researchers are investigating:
Brimonidine. One of the initial trials of neuroprotection looked at brimonidine as not only an IOP-lowering medication but also as a medication with a neuroprotective effect through its action on G-protein coupled receptor (GPCR) on RGCs. Although it is difficult to uncouple the neuroprotective and IOP-lowering effects, one study showed that both brimonidine and timolol had similar measurable IOP effects, but only brimonidine demonstrated preservation of visual fields.5 This still needs to be demonstrated in humans with repeatable prospective randomized clinical trials.
Glutamate receptors. An early neuroprotection trial looked at memantine, a selective non-competitive N-methyl-D-aspartate (NMDA) channel antagonist valuable as a treatment in Alzheimer’s disease.6 NMDA channels are ionotropic glutamate receptors, and elevated levels of glutamate can contribute to excitotoxicity and neurodegeneration.7 Memantine is able to block higher levels of glutamate that lead to excitotoxicity.7 Unfortunately, in the Phase III clinical trial, there was no convincing evidence of a significant effect vs. the control group with the primary outcome measure being visual field function.7 Presently, glutamate as a neuroprotective agent is currently just a hypothesis.8
Neurotrophic factors. These promising agents halt the loss of RGCs by stopping apoptotic pathways or inhibiting intracellular death signals.9 They act both in the central and peripheral nervous systems. Numerous classes of neurotrophic factors (NTF) modulate these pathways.10 The challenge has been identifying which are most useful to the RGCs and how to get them into the correct location with sustained delivery or continuous secretion.11 One theory on the advancement of glaucoma is that the increased IOP or other forces are blocking the transport of appropriate NTFs to the RGCs, causing cell death.
In addition to NTF discussed above and in clinical testing at Stanford, ciliary-derived neurotrophic factor (CNTF), brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GNTF) are all players in RGC survival in pre-clinical models.12,13 Limitations on delivery have included short in vivo half-life and low bioavailability due to these biologics’ larger molecular weight.14
One suggested solution is to insert a slow-release device intravitreally that will continue to release the factors; for example, using encapsulated cell therapy. One such device is the CNTF secreting device NT-501, currently in a Phase 2 clinical trial at the Byers Eye Institute at Stanford, Columbia University in New York and the Glaucoma Associates of Texas in Dallas (clinicaltrials.gov #NCT02862938).
We are surgically inserting this implant into patients and then following their course of glaucoma progression. With the insert sitting in the vitreous, it is slowly releasing CTNF on a continuous basis. We hypothesize that this will have ongoing effect and prolong RGCs’ survival and promote RGC function. Viral vectors have also been in development as a means to deliver CNTF and BDNF to target cells.15
Tumor necrosis factor-α. Another known contributor to glaucoma progression, TNF-α interacts with two main receptors, TNF-R1 and TNF-R2.16 TNF-R1 is centrally involved in apoptosis of cells, and it was shown to have elevated expression in an ocular hypertension model in rodents.16,17 Higher concentrations of TNF-α have been found in glaucomatous eyes vs. controls.18 Though this connection has been described and some immune-modulating drugs are approved for clinical use in humans with other diseases, no translational clinical trials target this particular pathway for glaucoma.
Heat shock proteins (HSPs). These are one component of cellular defense, and they assist in functions as chaperones, anti-apoptosis agents and signaling transducers. Types of HSPs exist in crystalline lenses, and rodent models show a neuroprotection and neuroregeneration effect with puncture of the lens capsule.19 A large cascade of activity was set off by the release of these proteins, including neuroinflammation and activation of glial components.20 We have not as yet developed a way to parlay this into clinical practice, but it is an interesting natural response to this incident and seems like a hopeful pathway for future development.
Nitric oxide (NO). This can mediate IOP-lowering effects through increase of aqueous outflow through the trabecular meshwork and Schlemm’s canal, the conventional pathway.21 However, it has been hard to find evidence of NO mediating neuroprotection of the ganglion cells.
One medication being investigated, nipradilol, is an alpha-beta blocker and donor of NO, an antagonist of alpha-1 and beta-1 and 2 adrenoreceptors. We know that nipradilol relaxes smooth muscle from the release of NO and also has antagonistic properties that inhibit muscle contraction. Research shows that glaucomatous eyes may have a baseline dysfunction in their NO pathways, creating decreased blood flow to the optic nerve head.22 Other studies demonstrate the slowing of RGC death in an optic nerve crush model in rats with nipradilol and also suggested that some of the neuroprotection may come from increased retinal blood flow properties.23,24 In addition, increased blood flow in the optic nerve head can result from treatment with nipradilol.25
There may be many benefits from incorporating NO or NO moieties into medical treatment for glaucoma. An exciting development in this area is the recent FDA approval of latanoprostene bunod, a molecule that acts as a prostaglandin analog and as an NO donor.
Stem cells. A common question patients ask me is if stem cells are able to restore their vision in glaucoma. This is a reasonable line of inquiry, since medicine has seen many advancements in the use of stem cells. While there is promise in this field, there are also substantial limitations. One of these limitations is getting those stem cells to connect with the brain itself.
The brain is a complex set of circuitry that is not easy to plug into, so we are a ways off on getting stem cells to take the place of dead or dying cells in the CNS. However, it does look like we can enhance survival of connected, living RGCs with stem cells.26 One study using intravitreally injected mesenchymal stem cells (MSCs) in rats resulted in prolonged survival of RGC axons. There was no effect on the RGC axons when the MSCs were injected intravenously.27 This was done in rats with glaucomatous optic nerve injury in a laser-induced ocular hypertensive model.
One mechanism by which stem cells would have beneficial effect on the RGCs is by continuous release of neurotrophic factors, which slow down the degeneration.28 Factors that can be secreted from stem cells include CNTF, fibroblast growth factor and GNTF.29 There may even be more factors released that have not yet been characterized. A concern some researchers have discussed: if we haven’t mapped all the possible neurotrophic factors that may be released, it is possible these stem cells could be secreting compounds that actually have deleterious effect on the optic nerve. Therefore, we should be sure we have fully characterized the NTFs that come from a line of cells before we use them.30
A New Hope
We’re living in a time of tremendous development in glaucoma research. Much of it is currently transitioning from animal models to human trials. What we should take from this and bring to the next glaucoma patient we have in our chairs is hope. We can confidently say that we are getting closer to a solution for them and their younger family members. I was happy to share that message with my patient. We don’t have the complete answer today, but I now know, more than ever, that we are taking appropriate steps toward bringing these treatments to the millions of patients that so desperately need them.
That is the excitement of neuroprotection research and, at some future stage we hope, neuroregeneration itself. Now, part of the thrill of treating glaucoma is the feeling that our treatment options—and patients’ prognosis for vision—are on the cusp of changing.
Dr. Groth is a clinical instructor of ophthalmology and glaucoma fellow at Byers Eye Institute at Stanford University.
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-7. |

