26th Annual Surgery Report- MIGS: Indications and Complications |
The management of postoperative ocular surgery patients is an important and growing part of optometric practice, regardless of practice modality. There are a multitude of benefits for a patient to continue their postoperative care with their primary optometrist, including but not limited to reduced travel costs and more personalized care. Additionally, in OD/MD group practices, shifting postoperative care to the optometrist opens the surgeon’s schedule to manage more complex cases. Let’s review how to manage some of the complications that may arise following a variety of anterior segment surgeries.
Cataract Surgery

|
|
Fig 1. Though rare, retained lens fragments may appear following uncomplicated cataract surgery. Click image to enlarge. |
At the one-day postoperative exam following this procedure, elevated intraocular pressure (IOP) is a concern. Although multiple factors can contribute to this complication, retained viscoelastic material is thought to be the major contributor.1
The elevation in IOP typically peaks at three to seven hours following cataract extraction, and will return to normal levels within 48 hours.2 Through use of topical IOP-lowering drops, oral acetazolamide or both, reduce the pressure in patients where it is elevated above an acceptable level. Also, take patient ocular history and optic nerve head cupping into consideration. Despite elevated IOP being benign for most patients, it may be sight-threatening for certain high-risk patients, and thus prompt and appropriate treatment is necessary.
For patients with pressure above 30mm Hg, consider sideport paracentesis for an immediate reduction in IOP. This involves applying pressure with a sterile instrument to release aqueous humor. The pressure has been shown to return to elevated levels within four hours. Add topical or oral hypotensives to ensure a sustained reduction.3,4
Patients are also at-risk for elevated IOP following the one-week post-op exam. These cases are most likely to be a steroid response, so either taper the steroids in these patients, stop them or switch the patient to an NSAID. Some cases may also require the addition of a topical hypotensive.
Postoperative cystoid macular edema (CME) following cataract surgery has been reported in 1% to 2% of cases.5 Pre-existing maculopathy and prostaglandin analog use put patients at an increased risk of developing CME.5 Identify CME with OCT in patients who do not correct to 20/20 with refraction and when the reduction in vision cannot be explained by other anterior or posterior segment findings.
Treatment for post-op CME involves the use of topical steroids and NSAIDs. Resolution may take four to 12 weeks with topical therapy.5 If there is no improvement with topical therapy, refer the patient to a retina specialist for evaluation and further management.
Although relatively rare, practitioners may note retained lens fragment in the anterior chamber following uncomplicated cataract surgery. One study identified retained fragments in less than 1% of routine cases.6 Most lens fragments can be identified on regular slit lamp examination, typically in the inferior angle (Figure 1). In cases with unexplained prolonged corneal edema or anterior chamber inflammation, perform gonioscopy to rule out a retained lens fragment. When noted, refer the patient back to the surgeon for decision on treatment with increased topical steroids vs. surgical removal.
During the first few weeks following surgery, patients may report a dark crescent or shadow temporally in their vision, which is typically related to the incision site. This visual phenomenon will fade and become less evident over the first month following surgery and eventually resolve. Less often, this can be secondary to negative dysphotopsias or even a retinal detachment. A negative dysphotopsia results from blockage of light on a portion of the retina and manifests as a dark crescent or curved shadow in the patient’s vision.7 In severe cases in which symptoms do not dissipate over time, consider referring back to the surgeon.
MIGS
The goal of glaucoma treatment is to prevent progressive optic nerve damage by lowering IOP. For years, the mainstay treatment for early to mild glaucoma was primarily drops or laser treatment. More recently, our armamentarium of glaucoma treatment options has expanded with the advent of minimally invasive glaucoma surgery (MIGS). Although MIGS procedures are effective in controlling glaucoma, they also carry a risk of visually threatening complications, and many patients require frequent follow-up visits.8
MIGS options have minimal tissue trauma, at least modest efficacy, rapid recovery and a high safety profile. MIGS can be categorized into six subgroups based on the mechanism of IOP reduction:8
(1) trabecular meshwork bypass by stent placement
(2) trabecular meshwork bypass by tissue excision
(3) increased aqueous outflow through Schlemm’s canal
(4) increased aqueous outflow through suprachoroidal space
(5) shunting aqueous outflow into the subconjunctival space
(6) reducing aqueous production by ciliary ablation
Although there are a number of different devices and types of procedures available, selection depends on patient and surgeon preferences.
|
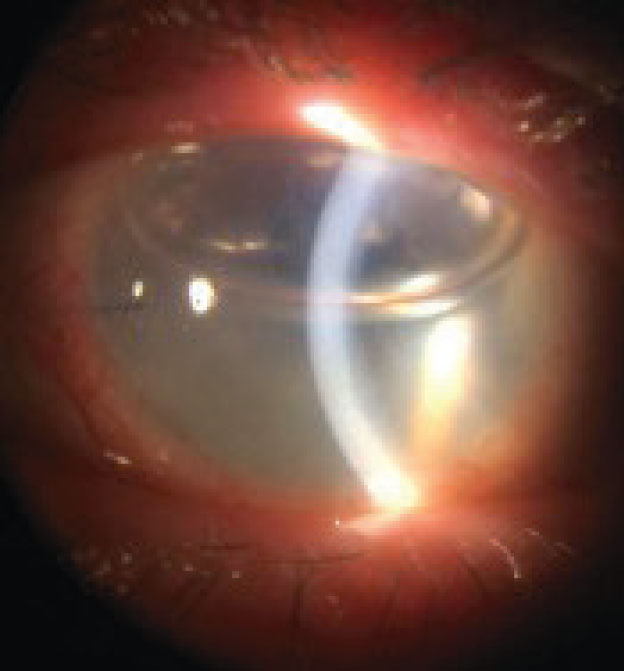
| Fig 2. Corneal edema may signal graft rejection post-PK. Click image to enlarge. |
The iStent (Glaukos) and iStent Inject (Glauckos) are indicated for patients with mild to moderate open-angle glaucoma. The first generation iStent is FDA-approved for ab interno placement in combination with cataract surgery.8 One trial noted that 72% of iStent patients maintained an IOP less than or equal to 21mm Hg compared to 50% in the control group, and 66% of iStent patients had a decrease in IOP of more than 20% without topical hypertensives compared with 48% for controls.8 Post-op complications associated with the iStent devices are well-reported, and the management of these complications can be applied to other MIGS procedures.
The most commonly reported complications associated with iStent placement include hyphema (up to 70%), stent obstruction (4% to 30%) and IOP elevation of >30mm Hg at a single visit or >10mm Hg above baseline (2% to 4.3%).8 Eyes receiving the iStent inject have demonstrated a greater absolute reduction in IOP while being medication-free.9 The most reported complications with iStent injection include IOP elevation or spike stent blockage or stent malposition and hyphema.10
When a stent is inserted into Schlemm’s canal, a blood reflux is expected and is an indicator of proper placement. Although excessive bleeding may reduce vision at the one-day postoperative exam and a micro-hyphema may be noted, the OD can monitor this complication without further intervention, as it is typically self-limiting. Patients with hyphema may also be started on a cycloplegic and advised to sleep with their head slightly elevated. Cases of severe or recurrent hyphema may require device removal.8
Manage IOP elevation following MIGS procedures with topical hypotensives, as the increase in IOP in these cases is typically transient. Once the patient’s IOP is under adequate control, reduce the number of meds. If the patient’s IOP remains elevated, perform gonioscopy to assess for proper placement of the stent and evaluate for any peripheral anterior synechiae or iris-stent obstruction. In a randomized, controlled trial for iStent, 4.3% of patients required repositioning of the stent because it was placed above or below the level of the trabecular meshwork.8
Pay close attention to gonioscopy to ensure proper positioning of the stent. Stents that are positioned more posteriorly and in patients with narrow angles are more likely to become occluded with iris. If stent obstruction is noted, refer the patient back to their surgeon for evaluation. Some cases of stent obstruction can be treated with nd:YAG laser while others may necessitate repositioning, removal or replacement.8 Surgical intervention to relieve stent obstruction or malposition may be necessary.8
When and how to reduce a patient’s topical medications following a MIGS procedure depends on the patient’s level of glaucoma and risk of progression. Usually it is best to discontinue prostaglandin analogs at the one-day post-op visit or substitute them with another topical hypotensive if the patient still requires IOP reduction.
Prostaglandin analogs can cause an increase in or slow the resolution of postoperative inflammation. Patients with mild glaucoma and low risk of progression can typically be taken off one of their medications at either the one-day or one-week postoperative exam. For those with moderate to severe glaucoma, the MIGS procedure often acts as an additive treatment, and reducing topical hypotensives or oral medications may not be indicated. If the patient is a steroid responder, wait until they are completely off of steroids for an accurate IOP assessment. Lastly, establish a new baseline for the patient following MIGs surgery to more effectively manage their glaucoma moving forward.
Penetrating Keratoplasty (PK)
|
| Fig 3. Post-EK, the surgeon will fill the anterior chamber with air to keep the graft adherent to the host cornea. Click image to enlarge. |
This transplantation involves the entire cornea and all of its layers. It is indicated in conditions such as advanced keratoconus, corneal degenerations and dystrophies, deep corneal scarring due to trauma or infection and corneal perforations.
Successful post-op management of PK starts with the patient understanding the expectations and limitations of the surgery. For PKs, a best-corrected visual acuity of 20/30 is considered successful. In most cases, patients will have high refractive error and astigmatism.
Patients must also understand that visual recovery will be a long, slow process, so they must be compliant with their post-op eye drops to prevent rejection. Eye drops are used post-surgically for at least one year or indefinitely in many cases.
The typical post-op ocular medication schedule post-PKs includes a topical steroid (e.g., prednisolone acetate 1%), topical antibiotic (e.g., moxifloxacin, besifloxacin) and an NSAID (e.g., ketorolac, bromfenac) (Table 1). This will vary depending on the surgeon and the medication used.
Corneal epithelial defects one-day post-op occur up to 67% of the time.11 Smaller defects generally resolve within a few days, but larger defects require additional management. For larger defects, consider a bandage contact lens or a temporary tape eyelid tarsorrhaphy to promote healing. Alternatively, use a cryopreserved or dehydrated amniotic membrane to speed up healing or in cases where the patient’s epithelial defect persists.
A key short- and long-term complication to monitor for is corneal graft rejection. This complication’s peak risk is within the first 1.5 years after transplantation.11 However, even grafts that are 20 years old may unexpectedly undergo rejection.
The most common sign of corneal graft rejection is corneal edema (Figure 2).11 Other signs to look for include white blood cells in the anterior chamber with or without hypopyon, endothelial keratic precipitates, neovascularization crossing the graft-vs.-host line, infiltrates and an epithelial or endothelial (Khodadoust) rejection line of white blood cells across the cornea.
It is imperative to discuss symptoms of rejection with the patient. An easy-to-remember mnemonic that is generally sufficient in education is “RSVP:” redness, sensitivity to light, vision loss and pain.
Loose or broken sutures can lead to irritation, corneal ulcers and, worst-case scenario, corneal graft rejection. Interrupted or running sutures can secure the corneal PK graft but may become loose or break over time. Remove any loose sutures that no longer serve their function promptly. The area near the base of the suture can also be at high-risk for development of a corneal ulcer. Locate a loose suture by staining the eye with sodium fluorescein (NaFl) and viewing it through a cobalt blue filter. The NaFl dye will pool underneath the loose suture.
Corneal graft rejection treatment involves the frequent use of a topical steroid eye drop (e.g., prednisolone acetate 1%, difluprednate), typically one drop every two hours, and an eventual taper once the eye shows signs of improvement. Depending on the severity, the patient may require concurrent oral steroids or a subconjunctival steroid injection.
Endothelial Keratoplasty (EK)
EKs are indicated in posterior corneal conditions such as Fuchs’ dystrophy, posterior polymorphous dystrophy, or endothelial decompensation from ocular surgeries. The most common are Descemet’s membrane endothelial keratoplasty (DMEK) and Descemet’s stripping automated endothelial keratoplasty (DSAEK).
In a DMEK graft, the endothelium and Descemet’s membranes are transplanted, while, in a DSAEK graft, the endothelium, Descemet’s membrane and the posterior stroma are replaced. Also, DMEK grafts have less endothelial cell loss long-term, greater visual quality and outcome and a lower risk for rejection.12-14 DSAEKs are reserved in more complicated cases, such as in eyes with preexisting tube shunts.
Table 1. Drop Schedule for Post-PK and -EK Management | ||||||
| Day 1 | Week 1 | Month 1 | Month 3 | Month 6 | Year 1 |
| Prednisolone acetate 1% | QID | QID | QID | TID | BID | OD OR B/C |
| Moxifloxacin 0.5% | QID | D/C | - | - | - | - |
| Bromfenac 0.07% | QD | QD | D/C | -0.42 | - | - |
As with PK grafts, successful management of EK grafts begins with patient education. After the transplant, the surgeon will fill up to 80% of the anterior chamber with air to keep the graft adherent to the host cornea. On the one-day post-visit, the surgeon will use less (Figure 3). Too small an air bubble raises the risk for graft detachment, but too much air may result in pupillary block and subsequent spike in IOP. Any eye with a bubble that covers 20% or less of the anterior chamber should be considered for a rebubble the same day. In order for an air bubble to function correctly, the patient must spend the majority of their time supine, with their eyes looking straight up, for several days after surgery. This needs to be reiterated to increase compliance and reduce complications.
Signs and symptoms of graft rejection in these cases are similar to those in PKs. Visualize graft detachments under the slit lamp using an optic section, focusing on the posterior cornea. Overlying sectoral stromal edema can help guide the clinician in identifying a graft detachment. Treatment includes rebubbling and ensuring compliance with supine positioning for the next three to five days. The post-op drop schedule for EKs is similar to that of PKs.
Postoperative surgical procedure management is a growing and important part of contemporary optometric practice. Be prepared to manage postoperative complications that are within your scope of practice and make appropriate and timely referrals back to the surgeon if and when surgical intervention is indicated.
Dr. Mackner completed his residency in ocular disease and surgical comanagement at Omni Eye Services in New York and New Jersey. He practices at Edina Eye Physicians and Surgeons in Minnesota.
Dr. Do completed his residency in ocular disease and surgical comanagement at Georgia Eye Partners in Atlanta. He currently practices at Kaiser Permanente Northwest in Portland, OR. He is a Fellow of the American Academy of Optometry.
1. Simon SS, Chee YE, Haddadin RI, Veldman PB, et al. Achieving target refraction after cataract surgery. Ophthalmology. 2014;121(2):440-4. 2. Kim JY, Jo M-W, Brauner SC, et al. Increased intraouclar pressure on the first postoperative day following resident-performed cataract surgery. Eye. 2011;25(7):929-36. 3. John M, Souchek J, Noblitt R, et al. Sideport incision paracentesis vs. anti-glaucoma medication to control postoperative pressure rises after intraocular lens surgery. J Cataract Refract Surg. 1993;19(1):62-63. 4. Ahmed II, Kranenmann C, Chipman M, Malam F, Revisiting early postoperative follow-up after phacoemulsification. J Cataract Refract Surg. 2002;28(1):100-8. 5. Henderson BA, Kim JY, Ament CS, et al. Clinical pseudophakic cystoid macular edema: risk factors for development and duration after treatment. J Cataract Refract Surg. 2007;33(9):1550-8. 6. Zavodni ZJ, Meyer JJ, Kim T. Clinical features and outcomes of retained lens fragments in the anterior chamber after phacoemulsification. Am J Ophthalmol. 2015;160(6):1171-5. 7. Mamalis N. Negative dysphotopsia following cataract surgery. J Cataract Refract Surg. 2010;36(3):371-2. 8. Yook E, Vinod K, Panarelli JF. Complications of micro-invasive glaucoma surgery. Curr Opin Ophthlamol. 2018;29(2):147-54. 9. Manning D. Real-world case series of iStent or iStent inject trabecular micro-bypass stents combined with cataract surgery. Ophthalmol Ther. 2019;8(4):549-61. 10. Popovic M, Campos-Moller X, Saheb H, Ahmed IIK. Efficacy and adverse event profile of the iStent and iStent inject trabecular micro-bypass for open-angle glaucoma: a meta-analysis. J Curr Glaucoma Pract. 2018;12(2):67-84. 11. Stechschulte SU, Azar DT. Complications after penetrating keratoplasty. Int Ophthalmol. 2000;40(1):27-43. 12. Feng M, Price M, Miller J, Price F.,Air re-injection and endothelial cell density in Descemet’s membrane keratoplasty: five-year follow-up. J Cataract Refract Surg. 2014;40(7):1116-21. 13. Guerra FP, Anshu A, Price MO, et al. Descemet’s membrane endothelial keratoplasty: prospective study of one-year visual outcomes, graft survival and endothelial cell loss. Ophthalmology. 2011;118(12):2368-73. 14. Anshu A, Price MO, Price FW. Risk of corneal transplant rejection significant reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119(3):536-40. |

