A 66-year-old white male, a longstanding glaucoma patient, presented for a standard follow-up exam with a complaint of slowly, progressively decreasing visual acuity.
 |
We’ve followed this patient in our office for more than 10 years for open-angle glaucoma OU. His glaucoma regimen has evolved during that time; currently he’s on Lumigan (bimatoprost 0.01%, Allergan) QHS OU. His intraocular pressure has averaged between 16mm Hg and 19mm Hg OU, with nominal hyperemia. Pachymetry readings were 544µm OD and 551µm OS.
Over the years, his neuroretinal rims and visual fields have remained stable OU. Heidelberg Retina Tomograph-3 (HRT-3, Heidelberg Engineering) imaging and OCT analyses of the retinal nerve fiber layer have not changed since we started measuring them 10 years earlier. Heidelberg Edge Perimeter (Heidelberg Engineering) visual fields confirmed a dense arcuate scotoma OU with a firm nasal step OD, which has also remained stable over time.
His visual decline was due only to cortical and nuclear cataracts OU. Ultimately, the decreased vision has begun to hamper his quality of life, and now it’s time to discuss cataract surgery.
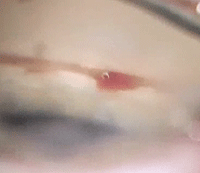 |
|
| Perioperative bleeding and postoperative inflammation are possible complications of the iStent (Glaukos). |
Diagnostic Data
Best-corrected visual acuity at this visit was 20/40- OD and 20/50- OS, although when we first met he had been 20/20 OD and OS through myopic astigmatic and presbyopic correction. Pupils were equal, round and reactive to light and accommodation with no afferent defect in either eye.
Medications consisted only of OTC vitamins and an occasional allergy medication as needed. He reported no allergies to medications.
IOP measured 16mm Hg OD and 17mm Hg OS with no other change to his glaucoma status described above.
Diagnosis
The patient’s crystalline lenses showed mild incipient nuclear sclerosis in both eyes. Accordingly, we made the appointment for his cataract surgery, first in the left eye and followed by the right eye.
Management
Our office protocol for cataract surgery is simple: Once the patient’s quality of life is negatively affected by reduced visual acuity, and we’re reasonably certain that lens extraction will significantly increase his BCVA and improve his quality of life (and of course, there are no extenuating ocular or systemic issues that would put the patient at risk), we’ll refer him to the most appropriate cataract surgeon, given the aforementioned considerations.
I’m a firm believer that the optometrist who’s been following the cataract patient for many years is very sensitive to the individual’s particular wants and needs and, in conjunction with the patient, is best positioned to offer this type of preoperative information to the surgeon in an effort to achieve excellent visual outcomes. I’ve discontinued the services of a few surgeons who made their own decisions based on their one, brief, pre-op consultation with my patient, only for the patient to have recurring issues postoperatively with the visual outcome.
In this vein, I sent an introductory letter to the operating surgeon introducing the patient and relaying pertinent information (in this case, a discussion of longstanding, well-controlled glaucoma), as well as information that pertains to the type of implant to be used and visual outcome goals following surgery (distance correction to plano OU).
Within a month, the patient underwent uneventful cataract surgery in the right eye.
When the patient presented for his one-week post-op visit, there was mild striate keratopathy, incisional bullous keratopathy as expected, and mild cells and flare in the anterior chamber of the right eye.
The single-vision IOL was well centered in the right eye, and the posterior capsule was clear. Visual acuity OD was 20/25+ and IOP was 17mm Hg. There were no retinal issues, and the patient was compliant with the normal post-cataract drop regimen and continued Lumigan OU. We scheduled him for a second follow-up in two weeks as a final clearance before surgery in the fellow eye a few days later.
At this second visit, the patient specifically asked me what his “pressures” were. He has always taken an active role in his care, and I thought nothing of it and told him (18mm Hg at this visit). He then said something to the effect of, “Looks like that thing they put in my eye to reduce pressure isn’t working.”
Somewhat surprised by this comment, I went back to the letter I received regarding the one-day post-op visit. (After the pre-op surgical consultation, as well as following the same-day post-op visit with the cataract surgeon, we receive letters outlining the surgeon’s significant findings.)
Sure enough, tucked in the middle of the letter—and, frankly, completely missed by me when I first perused it—was a note that an iStent (Glaukos) was implanted in the right eye during the cataract surgery.
Discussion
The iStent is part of a growing glaucoma surgical innovation known as minimally invasive glaucoma surgery (MIGS). The iStent is a small, snorkel-like device implanted through the trabecular meshwork during cataract surgery to allow more aqueous outflow. It essentially acts as an aqueduct that connects the anterior chamber to Schlemm’s canal. Because the majority of aqueous resistance through the trabecular meshwork complex likely comes from the juxtacanalicular tissue (JCT), creating a conduit through this tissue appears to relieve the intraocular pressure.1,2 Currently, the iStent is approved for implantation during cataract surgery in patients with open-angle glaucoma.
Because the device is being implanted into and through the trabeculum to Schlemm’s canal, a small amount of bleeding may occur at the time of surgery. Also, postoperative inflammation may be a bit more pronounced due to its intimate involvement with the uveal tract, and topical steroids may need to be adjusted accordingly. Generally, a wait of two or three months post-implantation is required to accurately assess the efficacy of the device in controlling IOP.
Cataract surgery in and of itself has been shown to decrease IOP by an average of 2mm Hg, and the initial results of iStent implantation indicate that it may further reduce IOP by an additional 3mm Hg.3 Although not a large decrease, that mild reduction in IOP may make the difference between a patient being treated with two instead of three medicines, or perhaps no medications instead of one. But will it result in an IOP reduction of 7mm Hg to 10mm Hg? Unlikely. So, although the device may reduce IOP by a few points, keep in mind the IOP-lowering effect you’re already achieving with the patient’s topical drops.
In our patient’s case, his treatment with Lumigan resulted in an average 7mm Hg to 10mm Hg reduction from unmedicated levels, and perhaps even more than that. Had his medication been able to stabilize him with just a 3mm Hg to 4mm Hg reduction in IOP, then I would have recommended the iStent from the get-go; however, given his longstanding stability and his medicated IOP reduction of 7mm Hg to 10mm Hg, the iStent would not likely have offered any significant improvement in his quality of life, so I didn’t recommend it in my referral letter.
You and I are the ones who handle the postoperative visual needs of the cataract patient, which means we need to step up to the plate in determining refractive outcomes. Likewise, you and I deal with the glaucoma patient’s postoperative situation, which means we need to be just as comfortable in making preoperative recommendations that affect the patient’s glaucoma postoperatively.
With that in mind, I called the operating surgeon to discuss how important it is that we should jointly decide on iStent implantation in future patients.
1. Rosenquist R, Epstein D, Melamed S, et al. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res. 1989 Dec;8(12):1233-40.
2. Johnson DH, Johnson M. How does non-penetrating glaucoma surgery work? Aqueous outflow resistance and glaucoma surgery. J Glaucoma. 2001 Feb;10(1):55-67.
3. Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, et al. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012 May;96(5):645-9.

