 |
History
A 45-year-old male presented to the office with an unsettling complaint; “My right eye is going blind!” He explained that his vision seemed to be gradually changing for the worse over the last couple of days.
He reported the left eye was unaffected, and claimed to experience no eye pain, redness, flashes, floaters or photophobia.
His systemic history was complicated, with hypertension for 15 years and kidney and liver cancer diagnosed two years earlier, with chemotherapy treatments ongoing. He also had a pituitary adenoma resection six years earlier. His medications included prednisone 5mg QD PO, Lovenox (enoxaparin sodium, Sanofi) and prochloperazine 10mg QD PO. He had discontinued his hypertensive medication, claiming that without it he was still adequately controlled.
He did, however, volunteer that his wife was filing for divorce, which was adding to his stress.
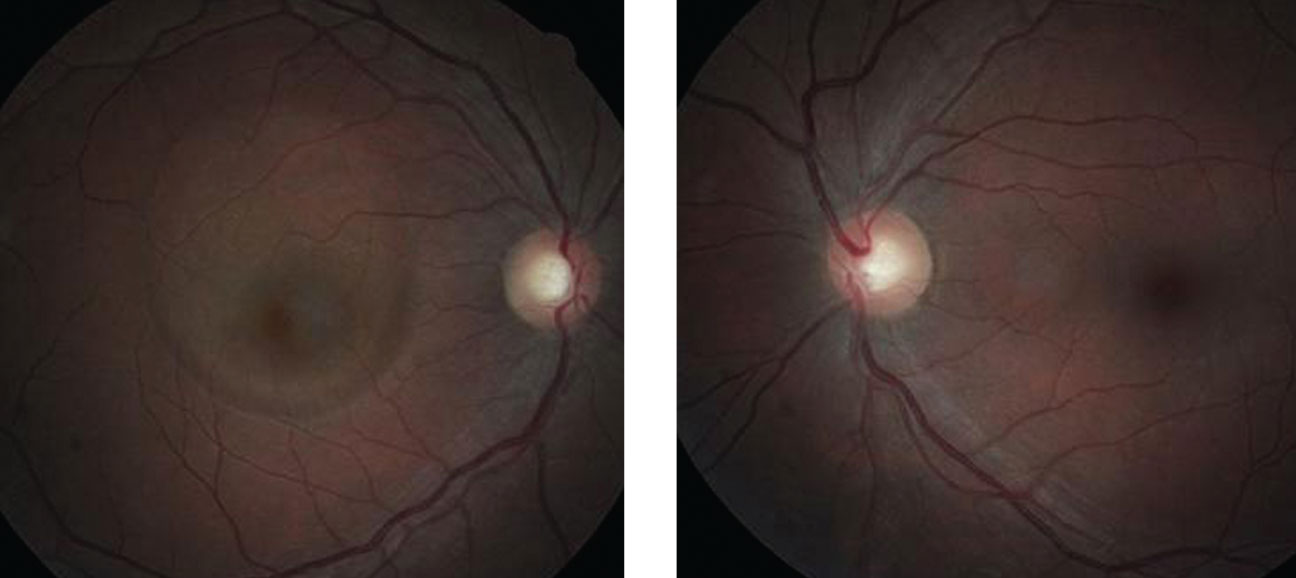 |
| Can these fundus images of the patient’s right (at left) and left eye uncover the cause of our 45-year-old patient’s reported gradual vision loss? |
Diagnostic Data
Best-corrected entering visual acuity was 20/40 OD and 20/20 OS, respectively, with no improvement upon pinhole. Pupils were equal and responsive to light with no evidence of afferent defect. Confrontation visual fields were full with mild distortion of the central face in the right eye.
His color vision and motilities were normal. The anterior segment was normal, with Goldmann intraocular pressures measuring 14mm Hg OU. The pertinent clinical observation is demonstrated in the photographs.
Discussion
Additional testing might have included a formal automated visual field, formal Amsler testing, photodocumentation and optical coherence tomography.
The diagnosis in this case is idiopathic central serous chorioretinopathy presumably, in part, secondary to the chronic oral steroid therapy for his pituitary malignancy.
Central serous chorioretinopathy (CSC) typically causes patients to experience sudden onset of distorted or blurry central vision. Patients have been documented to report central metamorphopsia, decreased color perception and even a relative central scotoma. The presentation is typically unilateral, although bilateral cases have been reported.1-3 There is typically no pain. Patients often have a history of using corticosteroids (topical, injectable, or oral), sympathomimetic agents, or medications for erectile dysfunction.1-9 Other contributory elements may include antibiotics, uncontrolled hypertension, alcohol, allergic respiratory disease and obstructive sleep apnea.2,10 Patients with CSC are typically younger. Most cases occur between the ages of 25 and 50.11 Men seem to be afflicted more frequently than women, with an incidence ratio of about 6:1.1,2,12,13 There are few if any racial predilections. Perhaps the most well-known association with CSC is the psychological profile known as “Type A” personality. These individuals, who are described as exhibiting the characteristics of time urgency, aggressiveness and competitiveness.16-19
Clinical evaluation of the patient with CSC reveals no external signs of ocular disease or inflammation. Mild hyperopic refractive shift (+1.25 or less) is often noted in the affected eye. Funduscopic examination shows a distinct, round or oval serous elevation of the macula with a loss of the foveal light reflex. An underlying area of RPE detachment may be seen concurrently in about 10% of patients.20,21 Associated findings can include cystoid macular degeneration, retinal atrophy and RPE tears (sick RPE or gutter syndrome), especially in chronic cases.22-24 There exists the possibility of choroidal neovascularization (CNV) as well.25,26 Cases involving CNV are typically associated with a poor visual outcome. Today, optical coherence tomography (OCT) is often used to confirm the diagnosis of CSC. OCT classically shows a bullous neurosensory retinal detachment from the underlying choroid, separated by an optically empty zone. Fluorescein angiography will typically demonstrate a focal point of fluorescein leakage under the serous detachment that gradually expands to fill the entire lesion; it is sometimes referred to as a “smokestack” or “ink blot” hyperfluorescent pattern.1
CSC remains incompletely understood. CSC appears to have a multifactorial etiology, with various systemic associations and a complex pathogenesis. The primary dysfunction appears to be localized ischemia and/or inflammation at the level of the choriocapillaris, which leads to hyperpermeability; this in turn results in decompensation of the retinal pigment epithelium, causing a focal detachment of the overlying neurosensory retina.19,20 Biochemical changes are likely at the root of this process. In patients with CSC, serum levels of catecholamines and glucocorticoids appear to be elevated, and this is believed to have a direct influence on the integrity of Bruch’s membrane.17-19,27 Based on these observations, it is reasonable to speculate that adrenergic receptors within the choroidal circulation are involved in the pathogenesis of CSC. Stimulation of adrenergic receptors often results in release of secondary messengers, (e.g., cyclic adenosine monophosphate) and this may produce the vascular or RPE changes that result in CSC.28
Most cases of CSC are self-limiting over a period of three to twelve months.1,11 The prognosis for visual recovery is excellent, with most regaining their pre-event acuity. Upon diagnosing the condition, any corticosteroid therapy should be immediately discontinued, if possible. A consultation with the patient’s primary care physician may be indicated in cases involving steroidal antiinflammatories for systemic conditions and steroidal inhalers for asthmatics. Fully 90% of CSC cases resolve spontaneously following the cessation of steroids.32 While the acute phase of CSC is usually self-limiting, the condition may be recurrent in as many as 50% of affected individuals.33 These patients often demonstrate cystic yellow lesions in the macula known as lemon-drop nodule. Lemmon drop nodules are indicative of mild RPE detachment. They may also stimulate RPE hyperplasia.
In non-remitting or recurrent cases, focal laser photocoagulation has been utilized in an attempt to arrest the leakage.34 Focal laser therapy does not necessarily ensure improvement in visual acuity; it merely hastens recovery and diminishes the likelihood of recurrence.1,34 There are risks associated with this treatment, most notably iatrogenic damage to the fovea and subsequent formation of CNV.1,25,26 For these reasons, most practitioners will employ laser therapy only in cases that fail to respond within a reasonable period of time, recurrent cases, or cases in which the patients are overtly symptomatic and insist on definitive treatment.
Photodynamic therapy (PDT) with verteporfin has also been used successfully in the treatment of CSC; it has been demonstrated to improve visual acuity, reduce leakage on fluorescein angiography, reduce subretinal fluid as demonstrated by OCT and foster choroidal remodeling with decreased choroidal permeability.34-36 Other experimental treatments for CSC have shown modest success, including anti-VEGF treatment and transpupillary thermotherapy.37,38 Oral therapy with corticosteroid antagonists, adrenergic receptor antagonists and carbonic anhydrase inhibitors (e.g. acetazolamide) has also been documented, but with limited efficacy.1
This patient was referred to retinology and a letter was sent to the general practitioner requesting some revision with regard to the oral steroids. The physician was receptive and alterations to the systemic treatment plan were made. The patient self resolved over the period of 6 weeks.
Dr. Gurwood thanks Julie Marsh, O.D. for contributing this case.
1. Sowka J, Gurwood A, Kabat A. Sowka, J.W., Gurwood, A.S., Kabat, A.G. The Handbook of Ocular Disease Management 12th Ed. Review of Optometry 2012; 147(4):1A–64A. 2. Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011;22(3):166-73 3. Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244-9. 4. Kleinberger AJ, Patel C, Lieberman RM, Malkin BD. Bilateral central serous chorioretinopathy caused by intranasal corticosteroids: a case report and review of the literature. Laryngoscope. 2011;121(9):2034-7. 5. Baumal CR, Martidis A, Truong SN. Central serous chorioretinopathy associated with periocular corticosteroid injection treatment for HLA-B27-associated iritis. Arch Ophthalmol. 2004;122(6):926-8. 6. Fernandez CF, Mendoza AJ, Arevalo JF. Central serous chorioretinopathy associated with topical dermal corticosteroids. Retina. 2004;24(3):471-4. 7. Koyama M, Mizota A, Igarashi Y, et al. Seventeen cases of central serous chorioretinopathy associated with systemic corticosteroid therapy. Ophthalmologica. 2004 Mar-;218(2):107-10. 8. Fraunfelder FW, Fraunfelder FT. Central serous chorioretinopathy associated with sildenafil. Retina. 2008;28(4):606-9. 9. Gordon-Bennett P, Rimmer T. Central serous chorioretinopathy following oral tadalafil. Eye (Lond). 2012;26(1):168-9. 10. Michael JC, Pak J, Pulido J, et al. Central serous chorioretinopathy associated with administration of sympathomimetic agents. Am J Ophthalmol. 2003;136(1):182-5. 11. Kloos P, Laube I, Thoelen A. Obstructive sleep apnea in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246(9):1225-8. 12. Marcuson J, Riley T. Central serous chorioretinopathy. Optometry. 2008;79(5):241-51. 13. Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008;115(1):169-73. 14. Todd KC, Hainsworth DP, Lee LR, et al. Longitudinal analysis of central serous chorioretinopathy and sex. Can J Ophthalmol. 2002;37(7):405-8. 15. How AC, Koh AH. Angiographic characteristics of acute central serous chorioretinopathy in an Asian population. Ann Acad Med Singapore. 2006;35(2):77-9. 16. Desai UR, Alhalel AA, Campen TJ, et al. Central serous chorioretinopathy in African Americans. J Natl Med Assoc. 2003;95(7):553-9. 17. Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc. 1986;84:799-845. 18. Wynn PA. Idiopathic central serous chorioretinopathy--a physical complication of stress? Occup Med (Lond). 2001;51(2):139-40. 19. Zakir SM, Shukla M, Simi ZU, et al. Serum cortisol and testosterone levels in idiopathic central serous chorioretinopathy. Indian J Ophthalmol. 2009;57(6):419-22. 20. Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond). 2010;24(12):1743-56. 21. Mudvari SS, Goff MJ, Fu AD, et al. The natural history of pigment epithelial detachment associated with central serous chorioretinopathy. Retina. 2007;27(9):1168-73. 22. Chang MA, Bressler SB. Photodynamic therapy for chronic pigment epithelial detachment in central serous chorioretinopathy. Can J Ophthalmol. 2009;44(2):221-2. 23. Iida T, Yannuzzi LA, Spaide RF, et al. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23(1):1-7. 24. Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002;133(6):787-93. 25. Shanmugam MP, Bhende M. Retinal pigment epithelial tears associated with idiopathic central serous chorioretinopathy. Indian J Ophthalmol. 2000;48(4):315-7. 26. Konstantinidis L, Mantel I, Zografos L, Ambresin A. Intravitreal ranibizumab in the treatment of choroidal neovascularization associated with idiopathic central serous chorioretinopathy. Eur J Ophthalmol. 2010;20(5):955-8. 27. Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (avastin) for choroidal neovascularization secondary to central serous chorioretinopathy, secondary to punctate inner choroidopathy, or of idiopathic origin. Am J Ophthalmol. 2007;143(6):977-983. 28. Sun J, Tan J, Wang Z, et al. Effect of catecholamine on central serous chorioretinopathy. J Huazhong Univ Sci Technolog Med Sci. 2003;23(3):313-6. 29. Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology. 2002;109(10):1765-6. 30. Giusti C. Association of Helicobacter pylori with central serous chorioretinopathy: hypotheses regarding pathogenesis. Med Hypotheses. 2004;63(3):524-7. 31. Figura N, Franceschi F, Santucci A, et al. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2010;15 Suppl 1:60-8. 32. Sharma T, Shah N, Rao M, et al. Visual outcome after discontinuation of corticosteroids in atypical severe central serous chorioretinopathy. Ophthalmology. 2004;111(9):1708-14. 33. Wong R, Chopdar A, Brown M. Five to 15 year follow-up of resolved idiopathic central serous chorioretinopathy. Eye (Lond). 2004;18(3):262-8. 34. Lim JW, Kang SW, Kim YT, Chung SE, Lee SW. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. Br J Ophthalmol. 2011;95(4):514-7. 35. Taban M, Boyer DS, Thomas EL, et al. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol. 2004;137(6):1073-80. 36. Chan WM, Lam DS, Lai TY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87(12):1453-8. 37. Schaal KB, Hoeh AE, Scheuerle A, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009;19(4):613-7. 38. Kawamura R, Ideta H, Hori H. Transpupillary thermotherapy for atypical central serous chorioretinopathy. Clin Ophthalmol. 2012;6:175-9. 39. Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992;81(4):379-86. 40. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103(12):2070-9; discussion 2079-80. |

