Trends and ControversiesCheck out the other feature articles in this month's issue: - A Simpler Way to Code Office Encounters |
The rising rates of myopia worldwide leave little up for debate—the condition is already considered a public health concern by the World Health Organization (WHO).1 But what to do about it is less clear. Most agree mitigating the spread of high myopia is a must, but what about low myopia? Will a lifetime correction of just -2.00D or -3.00D really make a difference in the long run for the patient’s ocular health and quality of life? A close look at the numbers and the host of possible long-term effects suggests the answer is yes.
By The Numbers
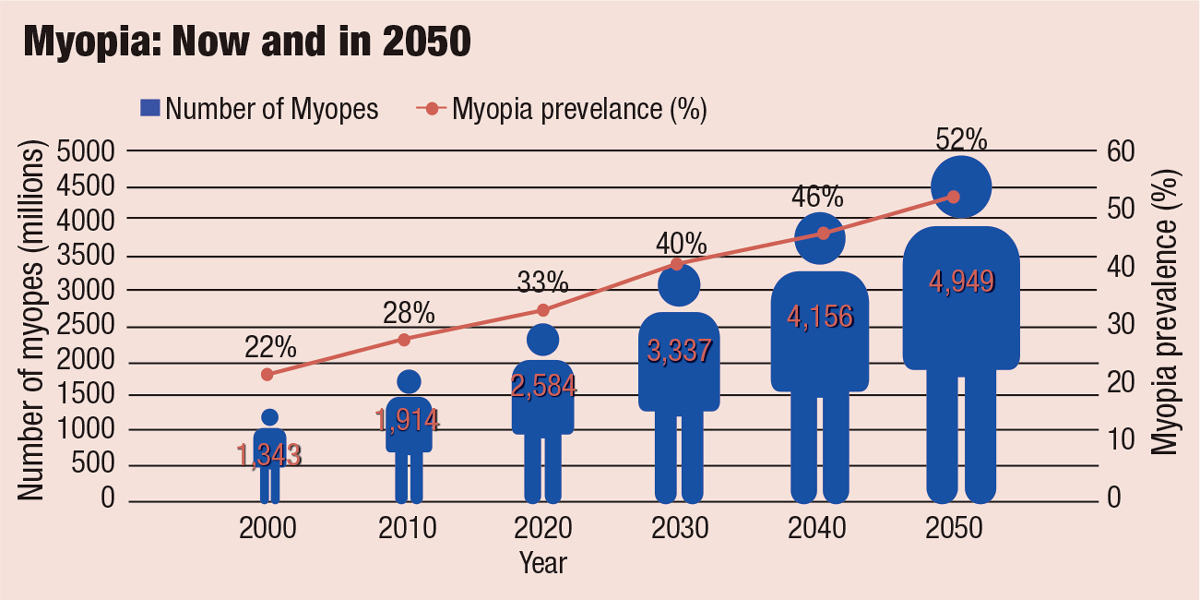
In 2015, the WHO, along with the Brien Holden Vision Institute, gathered top myopia researchers from around the world for a global scientific summit on myopia. The researchers noted that in 2010, myopia and high myopia were estimated to affect 28% and 3.0% of the world’s population, respectively. Current models project that by 2050, myopia and high myopia will reach epidemic proportions affecting 52% and 10% of the world’s population, respectively (Figure 1).1 Based on these projections, the WHO identified the increase in myopia as the number one health threat facing vision worldwide, in part because of its association with myopic macular degeneration and other conditions such as cataracts and glaucoma.1-3
 |
|
Fig. 1. The WHO estimates 52% of the world’s population will be myopic by 2050, up from just 22% in 2000. Adapted from: The Report of the Joint World Health Organization-Brien Holden Vision Institute Global Scientific Meeting on Myopia.1 Click image to enlarge. |
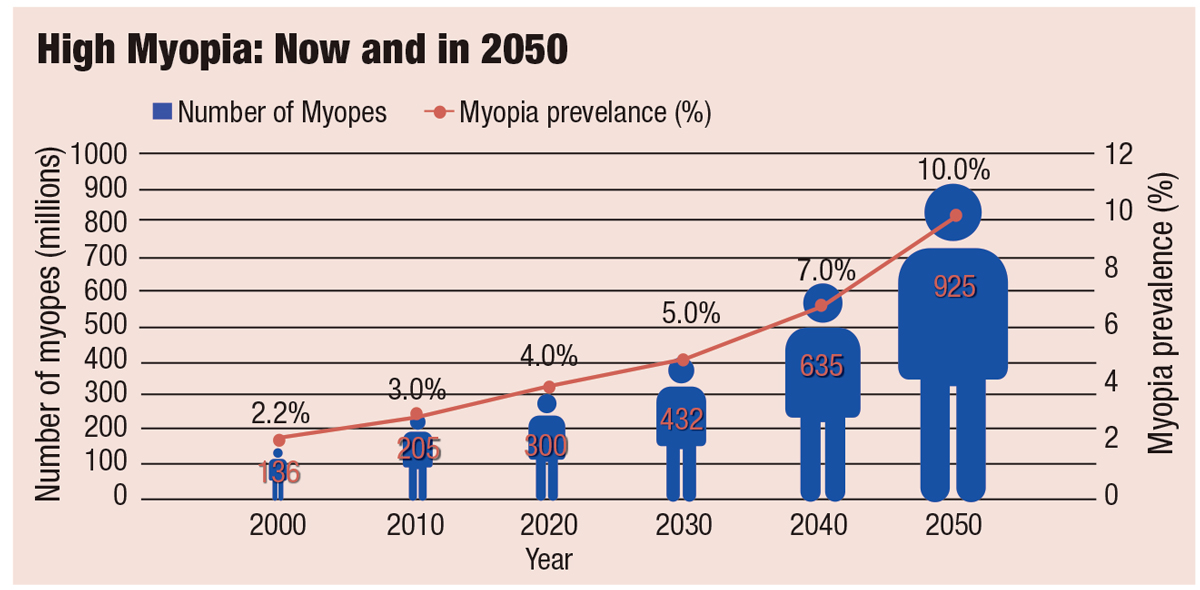
Rates of high myopia are on the rise as well (Figure 2).1 Although the definition of high myopia varies in the literature from -5.00D to a threshold as high as -8.00D—making analysis challenging—the WHO consensus recommends clinicians and researchers define it as -5.00D of myopia or worse.1 Children are being diagnosed at a much younger age now than in the past, and younger age at onset has been linked to faster progression and increased myopic severity.4,5
A study published in September 2020 also found that the age of onset significantly impacts the risk of high myopia in adulthood.6 Onset at age seven or eight led to a more than 50% risk of high myopia, and the risk drops with each year free of myopia. Patients with myopia onset at nine had a 30% risk, onset at age 10 had a 20% risk and onset at the age of 12 or older only had a 5% risk for progression to high myopia.6
Current research suggests myopia rates vary by population as well. Among late teenagers and young adults in Korea, Taiwan and China, for example, the prevalence is now between 84% and 97%.7
In the United States, one study found that the prevalence of myopia between -2.00D and -7.90D nearly doubled from 11.4% between 1971 and 1972 to 22.4% between 1999 and 2004.8 The same study found that the prevalence of high myopia, defined as more than -8.00D, increased eightfold, 0.2% to 1.6%, during the same period.7,8
An Environmental Issue
Although myopia develops as a complex interaction between environmental and genetic factors, environmental changes are believed to be the primary drivers behind the current myopia epidemic.9 Emmetropization, a visually guided process, depends on environmental exposures as a child. Myopia progression is due to elongation of the axial length, which is primarily due to the elongation of the vitreous chamber of the eye. Optical blur produced by a lag of accommodation or the eye’s response to accommodation may be what drives excessive growth.10,11
However, the visual processes at play with myopia development remain unclear, and research has yet to solidify a strong correlation with near work and the onset or progression of myopia.12
In fact, a recent study suggests the lag of accommodation develops concomitantly with, not prior to, myopia.13 The same researchers propose the involvement of the ON and OFF pathways in reading and myopia. While the natural environment is largely balanced between ON and OFF signaling, the team found reading dark text on a light background overstimulates the OFF channels and leads to reduced choroidal thickness within an hour. The opposite is true with light text on a dark background, which overstimulates the ON channels and increases choroidal thickness in one hour.13
Risk factors for myopia development include age, family history, race (Asian>Caucasian>Black), cycloplegic refraction at six years of age (<+0.75D increases risk of myopia later in life), near work and less time outdoors.9,14 A new study now suggests other environmental factors may increase the risk of myopia, such as the use of LED lighting when doing homework, dim light while performing near tasks, fewer sleeping hours, a consistent reading distance less than 25cm and living in an urban setting.14
Smartphone use was recently implicated as a possible risk factor, as school-aged children with myopia appear to use about twice as much data as their normal vision counterparts.15 Another study suggests less than three hours a week of physical activity and more than six hours a day of screen time can approximately double the risk of a teen developing or worsening myopia.16
Still, a literature review and meta-analysis published January 2020 found no significant association between screen time and myopia.17 The researchers speculate that reduced time outdoors, not increased screen time, might be more to blame for the myopia risk.17 The authors noted that myopia prevalence increased primarily with increasing education in urban Asia a few decades ago, not recently alongside increasing screen time. Yet another team found viewing electronic displays didn’t cause study subjects any more hyperopic defocus than the defocus caused by other stimuli.18
 |
|
Fig. 2. By 2050, high myopia is projected to reach 10% of the world’s population. Adapted from: The Report of the Joint World Health Organization-Brien Holden Vision Institute Global Scientific Meeting on Myopia.1 Click image to enlarge. |
Visual Impact
Worsening myopia comes with a number of drawbacks, all of which are proportional to the degree of myopia present, highlighting the importance of myopia control, even for low myopes.
Cost. The disease is associated with high financial costs to an individual, with one study finding a lifetime cost of as much as $17,020 for those who have myopia for 80 years.19 The mean cost per individual was approximately $709 per person per year and, not surprisingly, costs increased the earlier patients began wearing glasses. The Singaporean study noted the costs were driven by spectacles, contact lenses and optometry services, culminating in a total cost of approximately $755 million per year in Singapore.19
Impaired vision. Even if myopic patients are correctable to 20/20, their vision impairment can restrict their vocational options and provide them with poorer quality of life.
One study found patients with pathologic myopia experienced reduced quality of life and functional status in daily living compared with controls as a result of handicap, disability and lack of support.20 Even patients using vision correction experienced lower quality of life, the study found, and the average decrease in quality of life was -7.1% for LASIK patients, -13.0% for those using orthokeratology, -15.8% for spectacle wearers and -17.3% for soft contact lens wearers.20
Vision-threatening conditions. These are typically the result of excessive axial elongation, which leads to degenerative retinal and choroidal changes.1,21 Globally, high myopia is ranked second behind cataracts as the leading cause of correctable visual impairment, with 10% of all myopes having 6.00D of refractive error or worse.21 Myopic degeneration is the leading cause of blindness in Japan and the second leading cause of vision impairment in China and Denmark.22
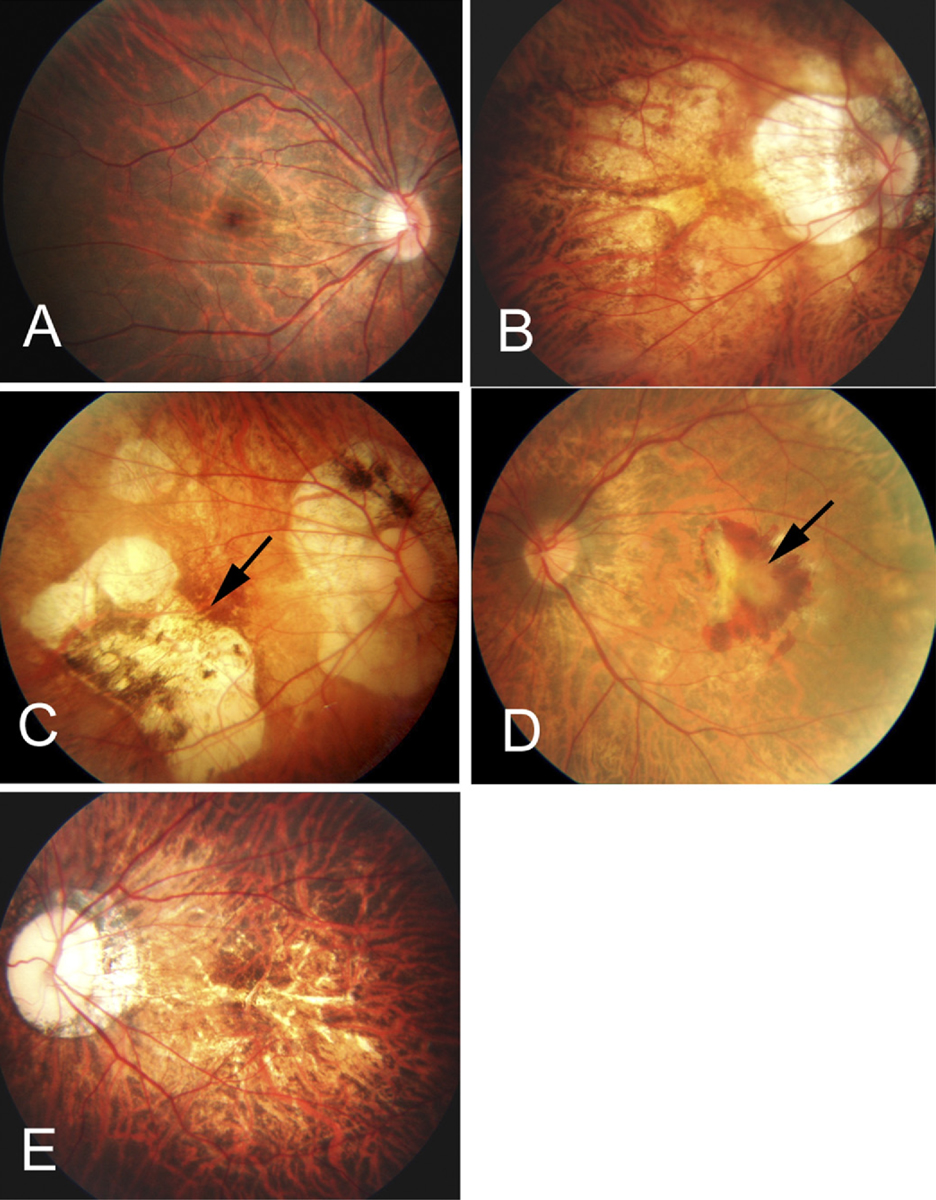 |
| Fig. 3. (A) The tessellated fundus of a 20-year-old, -14.00D myopic woman. (B) Diffuse chorioretinal atrophy seen in a 51-year-old, -21.00D myopic woman. (C) Patchy chorioretinal atrophy and a grayish-white, well-defined lesion (arrow) in a 35-year-old, -13.00D myopic male. (D) A macular hemorrhage and fibrovascular membrane (arrow) in a 67-year-old, 10.50D myopic woman. (E) Multiple yellowish lacquer cracks in a 28-year-old, -18.00D male myope. Reproduced with permission from: Hayashi K, Ohno-Matsui K, Shimada N, et al.32 Click image to enlarge. |
Myopic maculopathy is the most significant myopia-related cause of irreversible vision loss.1 Research suggests as many as 10% of pathologic myopia patients will develop this complication, of whom 30% will have a bilateral presentation.23
It is characterized by stretched blood vessels, peripapillary atrophy, posterior staphyloma, lacquer cracks in Bruch’s membrane, geographic atrophy of the retinal pigment epithelium and choroid, subretinal hemorrhages and choroidal neovascularization (Figure 3).23,24
One meta-analysis estimates that 10 million people globally had myopic maculopathy in 2015, of whom 3.3 million were blind.24,25 The researchers estimate that by 2050, visual impairment will grow to 55.7 million (one in 175), 18.5 million of whom will be blind.24,25
The risk of myopic maculopathy and its impact on public health are not limited to high myopes.24
Significant disease associations exist even at low levels of myopia.3,24 For example, patients with less than -5.00D of myopia contributed to 43% of the cases of myopic maculopathy in the Australian Blue Mountains Eye Study.24,26 There is no evidence of a safe threshold level of myopia for any of the known ocular diseases linked to myopia, including myopic maculopathy.3
A recent meta-analysis evaluated all observational studies performed between 1988 and 2019 related to myopia and found the condition is a risk factor for retinal detachments, primary open-angle glaucoma (POAG) and early and posterior subcapsular cataracts.27
The team found a prevalence of myopic macular degeneration of only 0.1% to 7% in patients with low myopia, but in as many as 65% of high myopes.27 While only a handful of studies investigate retinal detachment based on refractive error, the pooled analysis suggests an increased odds ratio of 3.45 for patients with any level of myopia, but as high as 12.62 for high myopes.27 The risk of cataract and POAG increased for all patients with myopia as well, and for high myopes in particular. Overall, the researchers found myopic patients had:27
- 100-fold higher risk of myopic macular degeneration
- Three-fold higher risk of retinal detachment
- Three-fold higher risk of posterior subcapsular cataract
- 1.59-times the risk of POAG
Based on this analysis, one in three high myopes is at risk of bilateral low vision with age.27
Why Control Matters
In a recent review, researchers used data from five population-based studies of the prevalence of myopic maculopathy to show that a 1.00D myopic increase was associated with a 67% increased prevalence of myopic maculopathy. The researchers further suggest that slowing myopia by 1.00D—regardless of baseline myopia—should reduce the risk of myopic maculopathy by 40%.24
According to myopia experts at the WHO Myopia World Summit, reducing the rate of myopia progression by 50% could reduce the prevalence of high myopia by up to 90%.1 And with a projected global prevalence rate of high myopia of 10.0% (925 million people) by 2050, the potential benefits are significant.1
Another study suggests that if a child could be kept from progressing from -1.00D to -3.00D, the risk of myopic maculopathy would decrease four- to five-fold, retinal detachment by three-fold and posterior subcapsular cataract by 1.5-fold.28
A study published in April 2020 analyzed 4,257 retinal detachment patients and 39,181 controls from the UK Biobank cohort. The researchers found that for each 6.00D increase in myopia, retinal detachment increased 7.2-fold. The study authors concluded that their results add weight to existing evidence suggesting that myopia control efforts may help prevent retinal detachments.29
Maintaining a patient at -1.50D instead of progressing to -5.00D or -6.00D is not only a matter of reducing the risk of axial elongation; it also significantly affects quality of life. Consider the patient who never progresses beyond -1.50D of myopia and can still drive without glasses or contacts. Keeping a patient functional without glasses is important, and a -1.50D myope is typically better able to navigate their surroundings than a -5.00D or -6.00D myope. Consider these scenarios: a patient loses their glasses during a car accident, or a hiker loses their glasses and has to navigate without them. Every diopter matters not only in visual acuity but also in their ability to function at different lighting levels.
 |
|
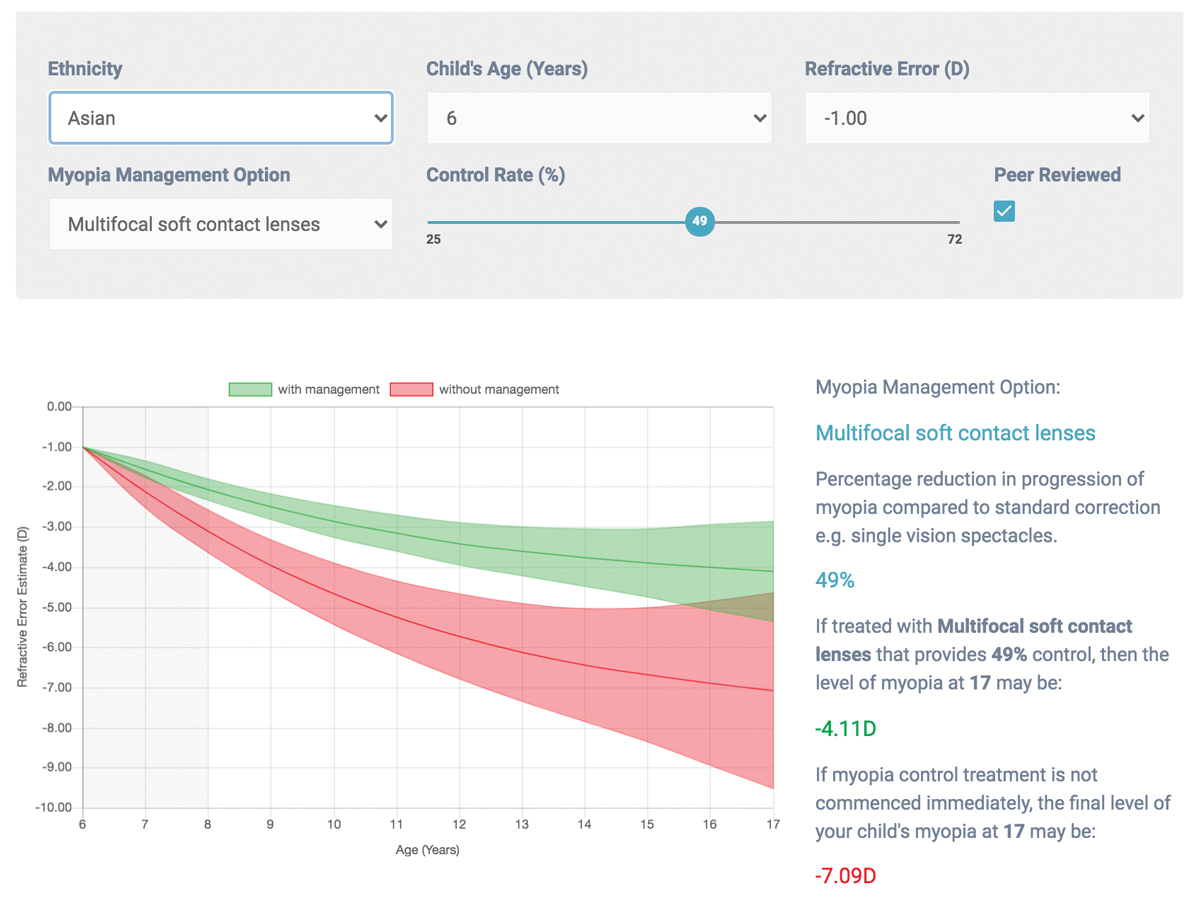
Fig. 4. The Brien Holden Institute’s free myopia calculator can help clinicians better understand the effects of myopia control strategies. Click image to enlarge. |
Researchers recently looked at the relationship between myopia severity and macular retinal thickness as it pertains to visual performance and found that visual acuity worsened progressively with dimmer lighting and higher myopia.30 The authors concluded that visual performance under photopic, mesopic and simulated night vision (with goggles) lighting conditions is influenced by both refractive error and retinal thickness.30 Thus, because visual acuity worsens progressively with dimmer lighting, going from -8.00D to -9.00D may not seem like a large jump as far as visual acuity, but the quality of vision will differ significantly based on lighting.30
Research on myopia is growing quickly, now showing that if Caucasian children diagnosed with myopia progress, on average, by -0.50D per year, then a six-year-old with -1.00D of myopia could be a -6.00D to -7.00D myope by the time they graduate from high school.9,31 Asian children progress even more rapidly, at an average of -0.87D per year.32 Of note: estimated progression rates are dependent on baseline age with decreasing progression rates as age increases.31 Intervening at age six could mean the difference between a final prescription of -2.00D to -3.00D compared with -6.00D or -7.00D, or even more depending on the child. One study shows reducing progression by 33% will keep 73% of myopic children below -5.00D—a threshold linked with an increased risk of choroidal neovascularization, retinal detachment, glaucoma and cataract.33
Available options to control the rate of myopia progression include low-dose atropine, multifocal soft contact lenses, orthokeratology and, in some countries, bifocal or multifocal eyeglasses. The Brien Holden Vision Institute provides a free myopia calculator that can help clinicians estimate the annual progression of myopia, showing the approximate refractive error that will result with and without myopia management (Figure 4). Clinicians can choose the management method, and based on probability and predicted efficacy of treatment, the calculator provides the predicted amount of myopia with and without treatment. This is calculated using the patient’s age, refractive error at presentation and ethnicity.
A Group Effort
Convincing a patient and their parents of the need to intervene now to prevent future risk takes strong conviction on the practitioner’s part. If clinicians do not advocate for the prevention of myopic progression, it will be almost impossible to prevent future vision loss from the increasing rates of myopia. Optometrists must take an interest, or patients will not.
Key points for patient education:
- Myopia rates are increasing at epidemic proportions, which is environmentally related.
- Every small increase in myopia is associated with a greater risk of permanent vision loss.
- By being proactive, rather than reactive, we have the opportunity to reduce myopia progression, which can improve quality of life and protect against disease risk.
- Slowing myopia by 1.00D should reduce the risk of myopic maculopathy by 40%.24
With more than half of the world’s population projected to be myopic by 2050, it is imperative that we heed the warning by the WHO and other proponents of myopia control—and treat myopia as a disease. This could make a significant difference in the lives of those with myopia, particularly those at higher risk of progression, including those of Asian descent, those with a higher refractive error at a young age and those who have myopic parents.
Dr. Poteet practices at TrueVision EyeCare in Acworth, GA, where she specializes in pediatric vision care. She currently serves as the president of the Ocular Wellness and Nutrition Society. She has a Master of Science in Human Nutrition and is a certified nutrition specialist.
1. The impact of myopia and high myopia: report of the Joint World Health Organization-Brien Holden Vision Institute Global Scientific Meeting on Myopia. University of New South Wales, Sydney, Australia, March 16-18, 2015. 2. Ohno-Matsui K, Lai TY, Lai CC, et al. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156-87. 3. Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012;31:622-60. 4. Chua SY, Sabanayagam C, Cheung YB, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016;36:388-94. 5. Donovan L, Sankaridurg P, Ho A, et al. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci. 2012;89:27-32. 6. Hu Y, Ding X, Guo X, et al. Association of age at myopia onset with risk of high myopia in adulthood in a 12-year follow-up of a chinese cohort. JAMA Ophthalmol. September 17, 2020. [Epub ahead of print]. 7. Holden BA, Wilson DA, Jong M, et al. Myopia: a growing global problem with sight-threatening complications. Community Eye Health. 2015;28(90):35. 8. Vitale S, Sperduto RD, Ferris FL. Increased prevalence of myopia in the united states between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009;127(12):1632-39. 9. Vera-Diaz F. Evidence based myopia control [video]. Cybersight, New England College of Optometry Myopia Control Center. January 11, 2018. 10. Ghosh A, Collins MJ, Read SA, et al. Axial elongation associated with biomechanical factors during near work. Optom Vis Sci. 2014;91:322-9. 11. Woodman EC, Read SA, Collins MJ, et al. Axial elongation following prolonged near work in myopes and emmetropes. Br J Ophthalmol. 2011;95:652-6. 12. Molly J, Smith MJ, Walline JJ. Controlling myopia progression in children and adolescents. Adolesc Health Med Ther. 2015;6:133-40. 13. Aleman AC, Wang M, Schaeffel F. Reading and myopia: contrast polarity matters. Sci Report. 2018;8:10840. 14. Grzybowski A, Kanclerz P, Tsubota K, et al. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020;20(1):27. 15. McCrann S, Loughman J, Butler JS, et al. Smartphone use as a possible risk factor for myopia. Clin Exper Optom. May 25, 2020. [Epub ahead of print]. 16. Hansen M, Laigaard P, Olsen E, et al. Low physical activity and higher use of screen devices are associated with myopia at the age of 16-17 years in the CCC2000 Eye Study. Acta Ophthalmol. September 9, 2019. [Epub ahead of print]. 17. Lanca C, Saw S. The association between digital screen time and myopia: a systemic review. Ophthal Physl Opt. January 13, 2020. [Epub ahead of print]. 18. Sah RP, Ramasubramanian V, Reed O, et al. Accomodative behavior, hyperopic defocus and retinal image quality in children viewing electronic displays. Optom Vis Sci. July 30, 2020. [Epub ahead of print]. 19. Zheng Y-F, Pan C-W, Chay J, et al. The economic cost of myopia in adults aged over 40 years in Singapore. Invest Ophthalmol Vis Sci. 2013;54:7532-37. 20. Chua SYL, Foster PJ. The economic and societal impact of myopia and high myopia. Updates on Myopia. Springer, Singapore; 2020:53-63. 21. Liu YM, Xie P. The safety of orthokeratology- a systematic review. Eye Contact Lens. 2016;42(1):35-42. 22. Buch H, Vinding T, La Cour M, et al. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004;111:53-61. 23. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2003;87:570-3. 24. Bullimore MA, Brennan NA. Myopia control: why each diopter matters. Optom Vis Sci. 2019;96(6):463-65. 25. Fricke TR, Jong M, Naidoo KS, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 to 2050: systematic review, meta-analysis, and modelling. Br J Ophthalmol. 2018;102:855-62. 26. Vongphanit J, Mitchell P, Wang JJ, et al. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2010;117:1763-8. 27. Haarman AE, Enthoven CA, Tideman JWL, et al. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. 28. Gifford P, Gifford KL. The future of myopia control contact lenses. Optom Vis Sci. 2016;93:336-43. 29. Han X, Ong JS, An J, et al. Association of myopia and intraocular pressure with retinal detachment in European descent participants of the UK Biobank cohort: a Menedlian randomization study. JAMA Ophthalmol. April 30, 2020. [Epub ahead of print]. 30. Tan CS, Li KZ, Tan M, et al. Relationship between myopic severity and macular retinal thickness on visual performance under different lighting conditions. Ophthalmology Retina. 2017;1:339-46. 31. Donavan LA, Sankaridurg P, Ho A, et al. Rates of myopia progression in children. Invest Ophthalmol Vis Sci. 2010;51:1694. 32. Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117:1595-1611. 33. Brennan NA. Predicted reduction in high myopia for various degrees of myopia control. Cont Lens Anterior Eye. 2012 Dec;35(Suppl):e14-e15. |

