| About this Series To help optometrists strengthen their protocols for managing conditions that require ongoing—perhaps life-long—care, this series explains the steps to take after confirming a diagnosis, from day one through long-term management. Each installment in the five-part “Now What?” series will cover a different chronic condition: My Patient Has Recurrent Corneal Erosion...Now What? |
As the leading cause of vision loss in adults older than age 60 in the United States, age-related macular degeneration (AMD) is unavoidable in optometric practice.1 The initial diagnosis, which can be alarming for many patients, requires significant patient education, baseline evaluation and correspondence with other healthcare providers. Here, we discuss a strategy for addressing these first-visit obligations.
Patient Education
As is true of many ocular conditions, patients may or may not know anything about AMD upon diagnosis—making patient education just as individualized as the management plan. Many are completely unfamiliar with the condition, and even those who do know about AMD may have inaccurate information. In addition, some who have family members affected by AMD may have increased anxiety about suffering vision loss themselves. Thus, patient education at the initial diagnosis can require a significant amount of chair time, as you should address these topics with the patient and their family members:
What is AMD? Macular degeneration is a degenerative condition of the retina that specifically affects the macula. The macula is responsible for central, detailed vision and is required for tasks such as reading or driving. Macular degeneration is the most common cause of vision loss in patients older than 60, but not all patients with the condition will suffer from significant vision loss. Furthermore, contrary to many laymen’s notion, AMD does not typically cause “total blindness,” a point that needs to be emphasized during initial patient education.
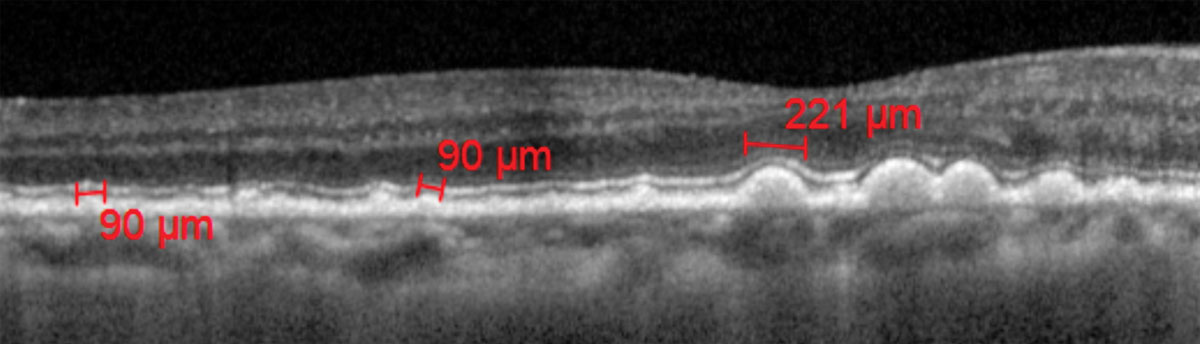 |
| Fig. 1. Drusen size measured with calibers on OCT. Click image to enlarge. |
What does dry vs. wet AMD mean? All patients with AMD begin with the non-neovascular, or dry, form. This may go undetected or undiagnosed. According to a recent study, 33% of patients who have AMD may go undiagnosed during an eye examination.1 About 10% of patients with dry AMD will develop subretinal or choroidal neovascular membrane (CNVM), a secondary complication where blood vessels grow from underneath the retina, leaking fluid and possibly bleeding. This complication can cause sudden and dramatic visual decline.
CNVM is associated with other macular diseases and is not unique to AMD. However, because of the fluid leakage, this neovascular stage of AMD is commonly known as “wet” or “exudative” AMD. CNVM often requires treatment by a retina specialist. The sooner wet AMD is detected, the better the visual outcome can be. Although past data reported that 90% of patients with wet AMD had significant vision loss, today’s treatments can help many patients with wet AMD maintain reasonably good central vision. Currently no treatments exist for dry AMD, and 10% of patients are at risk for significant vision loss due to geographic atrophy (GA), a process in which the retinal pigment epithelium and photoreceptors essential for vision are lost due to degeneration.2
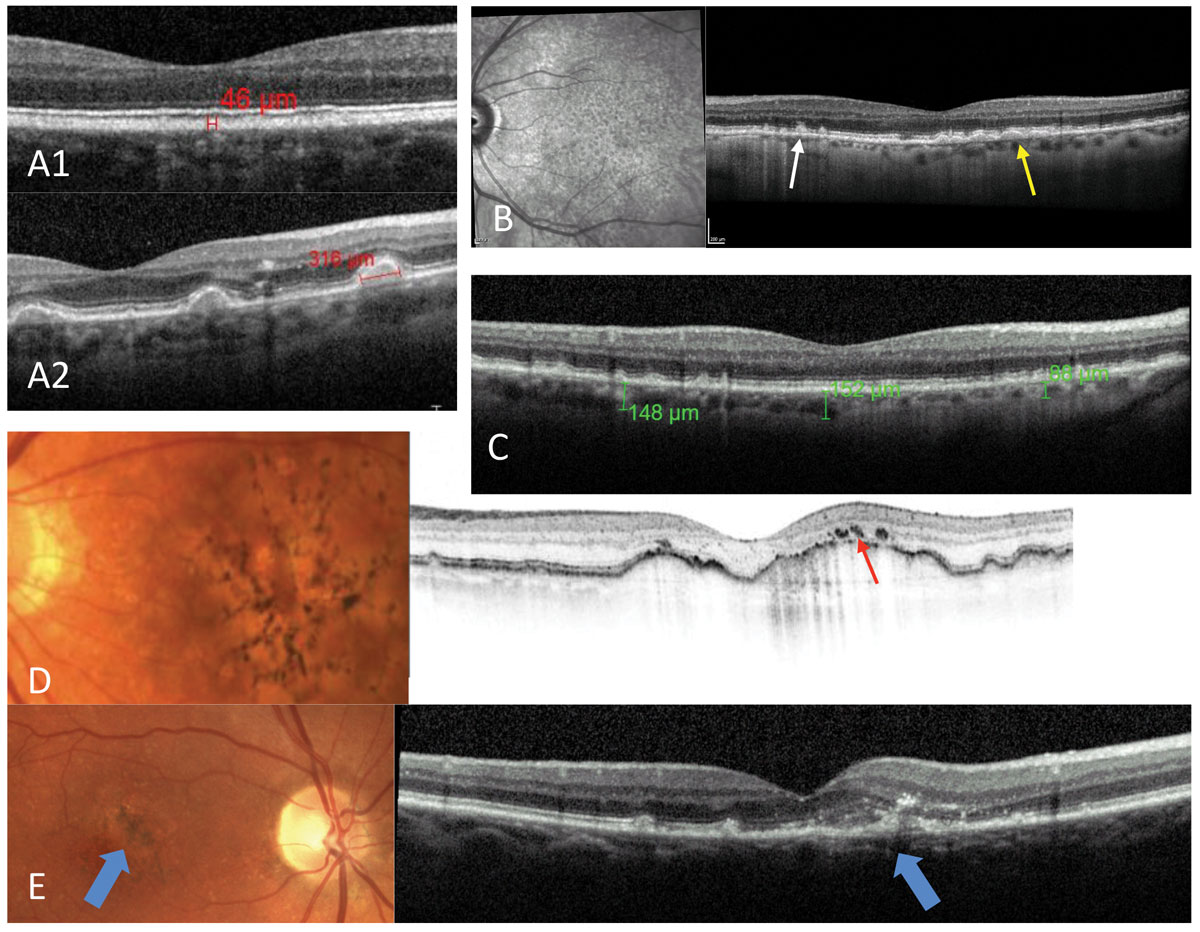 |
| Fig. 2. Possible OCT findings in AMD: small drusen (A1), large drusen (A2), presence of both typical sub-RPE drusen (yellow arrow) and RPD, with lipofuscin deposits above the RPE (white arrow) and the pattern of RPD discernable on the infrared photo (B). Thinner than average choroidal thickness consistent with AMD (C). Pigmentary changes over large pigment epithelial detachments (red arrow) (D). Early detection of CNV (blue arrows) (E). Click image to enlarge. |
What are the risk factors and what can I do to change them? Tobacco use is the number one modifiable risk factor, as smoking increases both the risk of developing AMD and the risk for progression of the disease.3,4Sunlight exposure over a lifetime contributes to the development of AMD, and appropriate protection with sunglasses and hats can limit the exposure.5 In addition, a healthy, balanced diet high in brightly colored fruits and vegetables and green leafy vegetables may delay the progress of AMD.6
The overall health of the body is important for the health of the eyes, as research links regular exercise to a lower risk of AMD development and progression, and conditions such as high cholesterol, hypertension and obesity can all contribute to AMD.7-10 Clinicians can recommend vitamins high in antioxidants and pigments important for the health of the macula for patients at certain stages of macular degeneration.11 Patients under medical care and taking multiple medications should discuss supplementation and any dietary modifications with their primary care physicians.
 |
| Click table to enlarge. |
Should I tell my children and siblings? In addition to modifiable environmental factors, research has identified many genes in association with AMD. Because an inheritance tendency exists for AMD, family members may benefit from lifestyle modifications and routine eye examination.12 Genetic testing for patients with AMD and those at risk remains controversial.13 Although commercially available genetic testing is marketed to tailor vitamin supplementation to a patient’s genome, contradicting study results have thwarted any consensus on whether or not genetic profile alters response to vitamin supplementation.14-17
How can macular degeneration affect my vision? AMD can lead to varying levels of vision loss including legal, but not total, blindness. The disease affects the central vision, therefore reducing our ability to read, drive or perform other tasks requiring detailed central acuity. In addition, patients with AMD may notice that they do not adapt as quickly to sudden changes in lighting conditions, as delayed dark adaptation is one of the earliest functional symptoms of AMD.18 Therefore, it may take more time for patients to adjust to both bright and dim illuminations. They may require more light for reading and seeing detail, as well as increased contrast in reading materials.
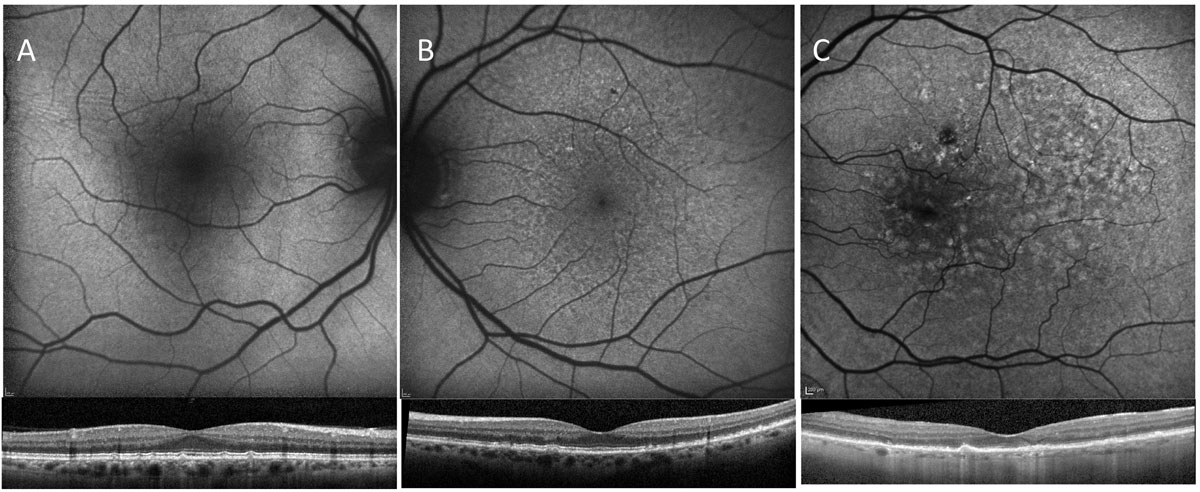 |
| Fig. 3. The top FAF images correspond with the OCT B-scans below. With OCT imaging, all of these patients have presence of intermediate sized drusen; however, patient A has much less disruption of the autofluorescent signal than patients B and C, suggesting the overall health of the RPE in patient A is better than that of B and C. Click image to enlarge. |
Not all patients with AMD have significant vision loss. While central vision is important for detailed tasks, the peripheral vision remains intact in patients with AMD. Therefore, most patients will not require assistance for most of their daily living activities.7 Still, it’s important to remind all AMD patients, even those with only mild visual impairment, that low vision interventions can help improve their visual performance both now and as the disease progresses, if they so desire.
Day One Responsibilities
Clinicians should complete a careful patient history at the initial visit to identify modifiable risk factors such as tobacco use, poor diet, significant sunlight exposure and systemic factors such as hyperlipidemia, hypertension and obesity. Communication and comanagement with the primary care physician improves outcomes by addressing concerns such as tobacco cessation, dietary modifications and control of contributing systemic disease.
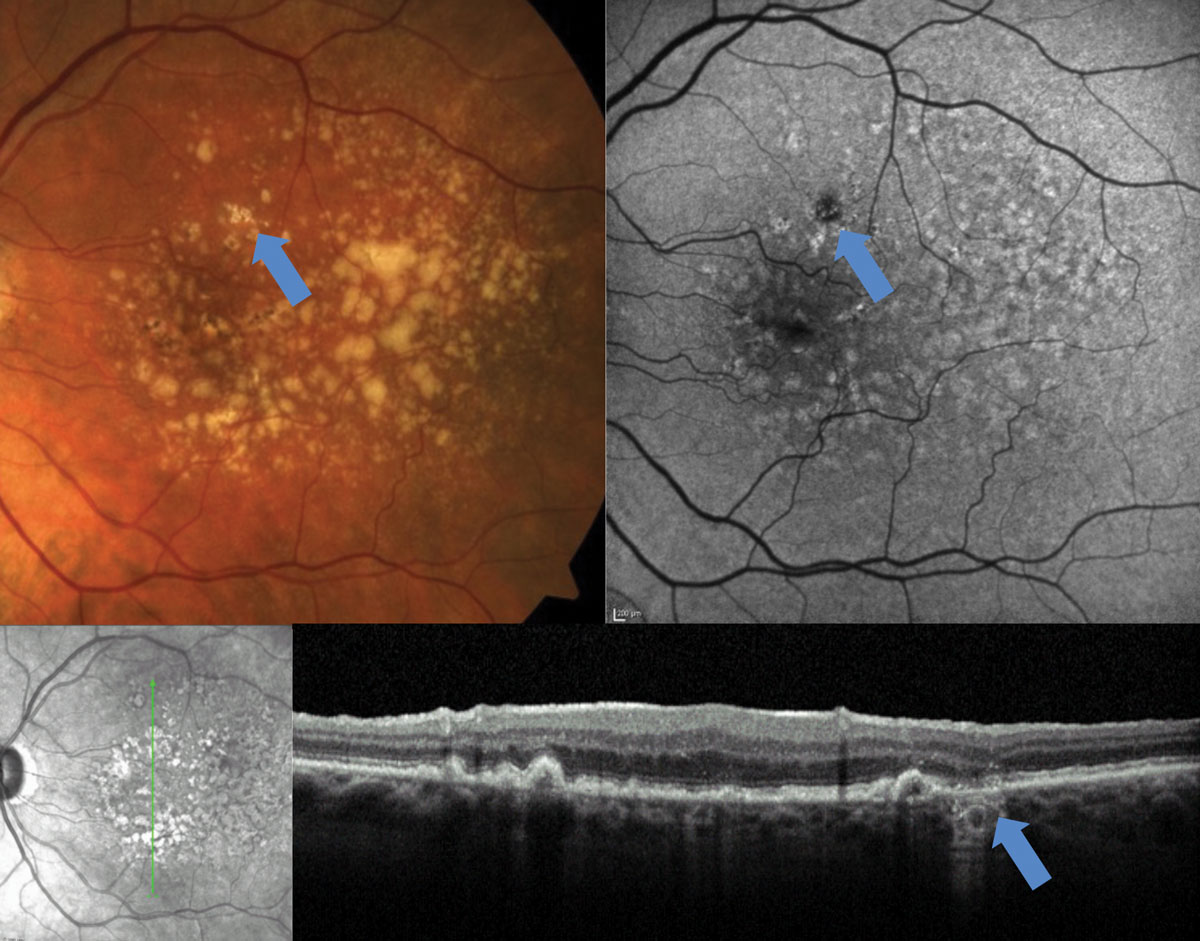 |
| Fig. 4. This early area of GA, identified with FAF and OCT, would be difficult to detect on fundus examination. Click image to enlarge. |
The first clinical steps after the initial diagnosis include determining the stage of the condition, identifying any retinal findings that increase the risk of progression to advanced stages and ruling out the presence of CNVM.
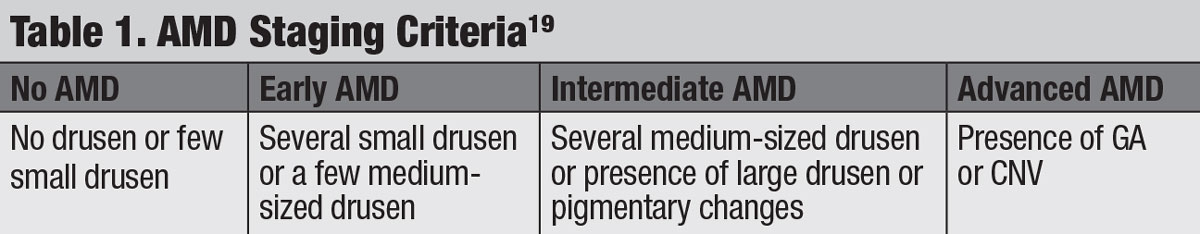
Staging. This is key because the Age-Related Eye Disease Studies (AREDS1 and AREDS2) found that those with intermediate AMD benefited from vitamin supplementation.11 Generally accepted staging data, simplified from the AREDS, categorize small drusen as ≤63µm, medium drusen as >63µm but ≤125µm and large drusen as ≥125µm (Table 1).19 The caliber of large retina veins near the optic disc is approximately 125µm, which can be used as a reference to estimate the size of drusen.20 But more practically, small drusen are difficult to detect clinically; thus, easily discernible drusen can be considered intermediate, and quite obvious drusen can be considered large. Fundus findings of large soft drusen and pigmentary changes also increase the risk of progression to advanced stages and should be noted.
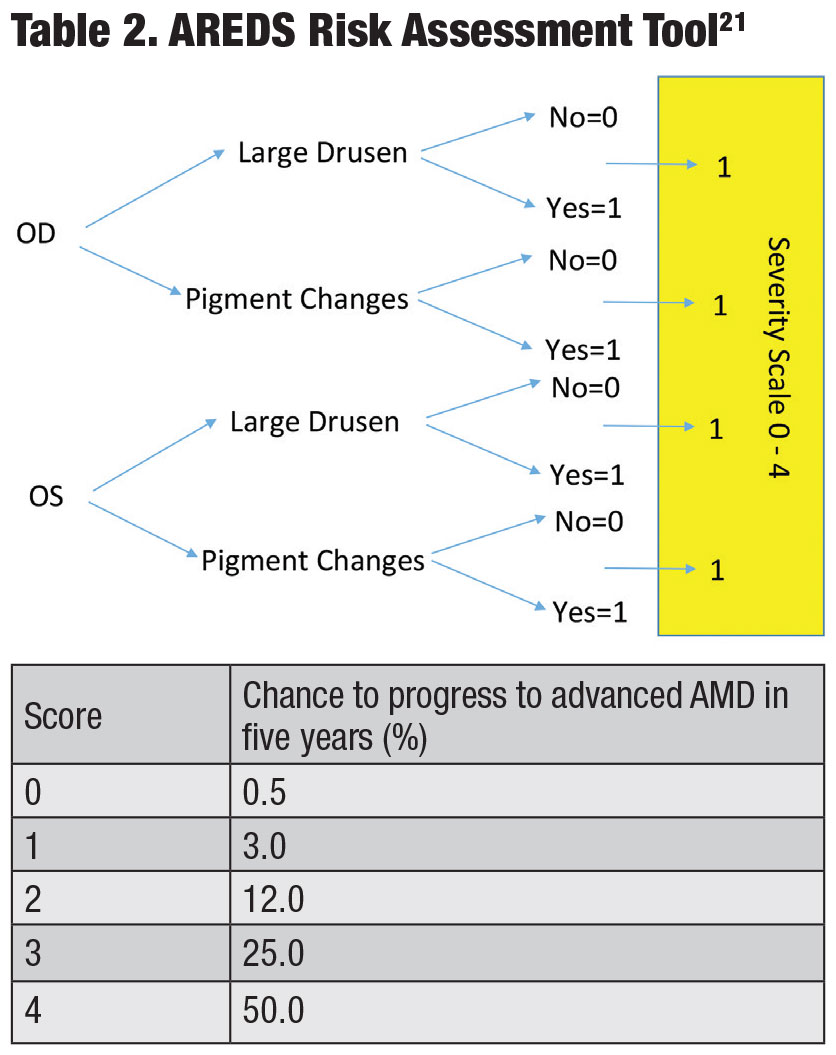
Data from AREDS1 and 2 provide a simple dry-to-wet AMD risk assessment tool that assigns a point for each finding of large drusen and pigmentary changes per eye (Table 2).21 The risk is calculated based on the patient’s point score (0 points = 0.5% risk of conversion to advanced AMD in five years, 1 point = 3.0% risk of conversion, etc.). Optical coherence tomography (OCT), among other instruments, can also be a useful tool for determining drusen size (Figure 1).
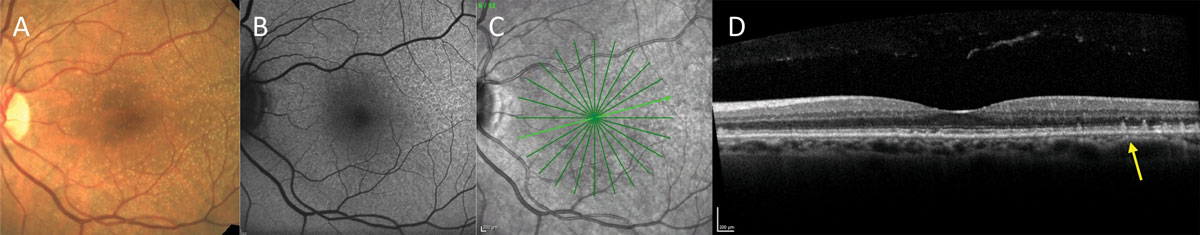 |
| Fig. 5. Fundus photo (A), FAF (B), infrared image (C), and OCT (D) of a patient with small to intermediate size drusen on clinical examination. No pigmenatry changes or large drusen are present, suggesting the risk for progression to advanced stages is low. However, FAF and OCT reveal the presence of RPD, which is known to carry increased risk of advanced AMD. Click image to enlarge. |
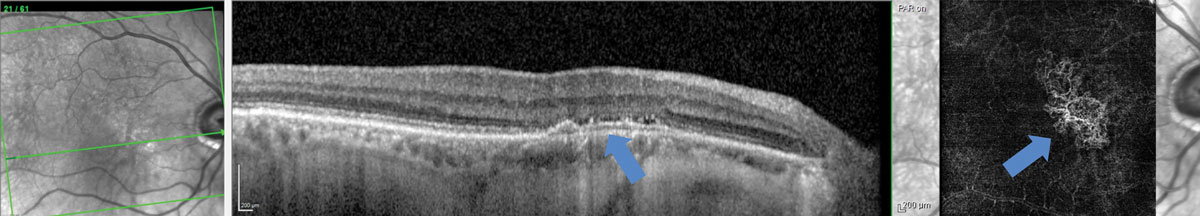 |
| Fig. 6. Type 1 sub-RPE choroidal neovascular membrane with minimal evidence of exudation on OCT but evidence of neovascular net in the avascular region on OCT-A. Click image to enlarge. |
Progression and CNVM assessment. Fundus and additional diagnostic examination should also be performed to carefully evaluate for signs of CNVM. During the fundus examination, the presence or absence of subretinal fluid, hemorrhage or both should be documented, as these signs may indicate the presence of CNVM.
Diagnostic imaging with OCT and fundus autofluorescence (FAF) should be considered at the initial visit to more thoroughly evaluate both the risk of progression and the presence of CNVM. It can also help establish a visual baseline of the disease state, thereby serving as a valuable resource to monitor a patient’s progression or efficacy of treatment.
 |
| Click table to enlarge. |
In addition to evaluating drusen size, OCT scans can also help determine choroidal thickness (patients with AMD tend to have thinner than average choroids); pigmentary changes; presence of reticular pseudodrusen (RPD), which carries increased risk of progression to advanced stages; and presence of sub- or intraretinal fluid and CNVM (Figure 2).22-25
FAF can assist in the detection of RPD and early GA and can reflect the general status of the retinal pigment epithelium (Figures 3-5).26 The additional information gleaned by OCT and FAF provides a more complete clinical picture than fundus evaluation alone as to the overall risk of progression to advanced stages.
Additional testing such as OCT angiography (OCT-A), intravenous fluorescein angiography (IVFA), or indocyanine green angiography (ICGA) may be necessary if there is suspicion of CNVM with fundus evaluation or OCT imaging.27 Patients with evidence or suspicion of CNVM require prompt referral to a retina specialist for additional imaging and possible anti-vascular endothelial growth factor treatment (Figure 6). Because exudative AMD is a chronic disease in most cases, conversion to this stage often requires continued care and comanagement between the referring doctor and the retina specialist.
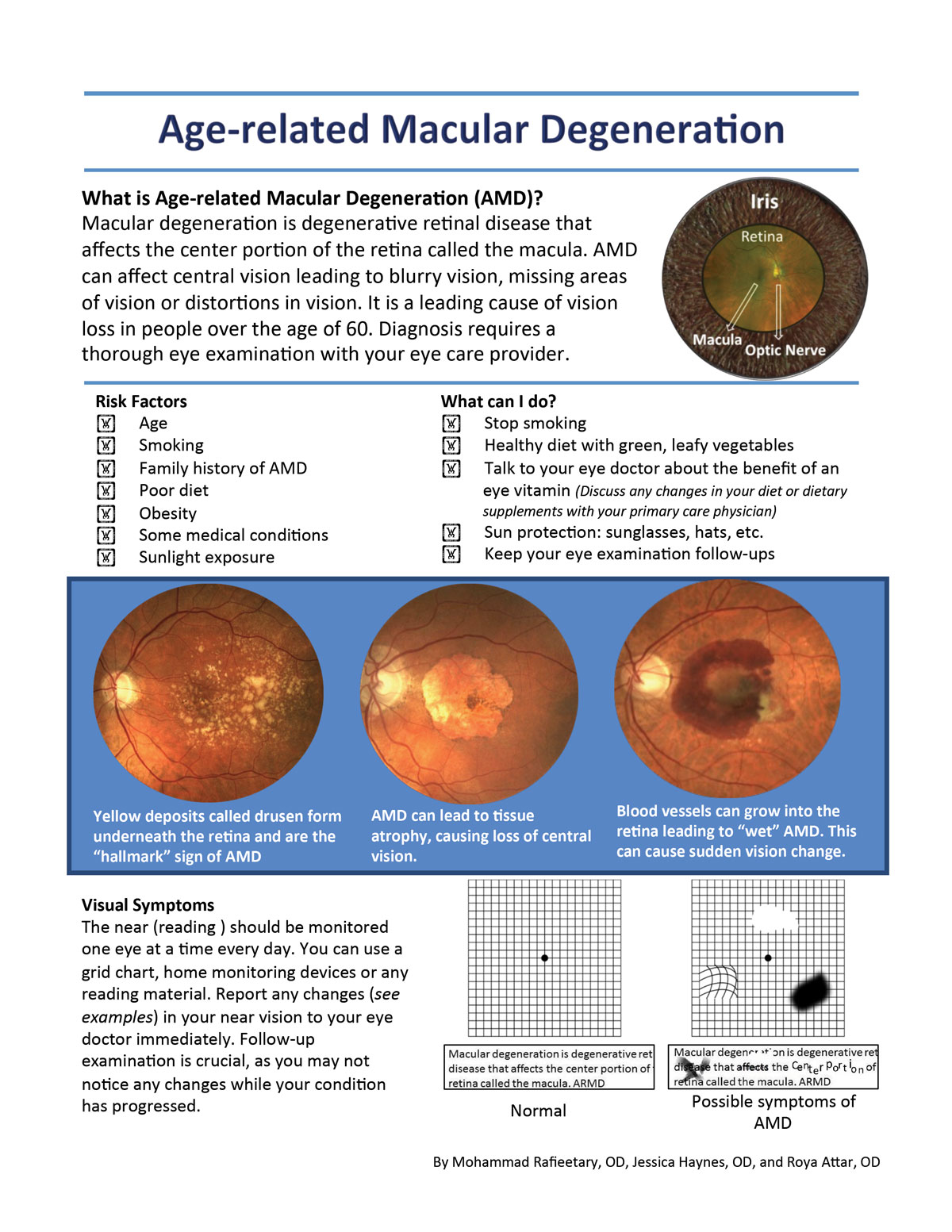 |
| Click image to download this patient handout. |
Assigning Homework
With all of the clinical testing out of the way, patients should be counseled on the importance of at-home monocular vision monitoring, as it can lead to earlier detection of CNVM and better visual outcomes.28 Clinicians should demonstrate tools such as the Amsler grid, or other visual target, with specific instructions on daily vison monitoring, including what changes to look for and steps to take if the patient detects a change. In addition, remote home monitoring devices are now available that can detect early visual changes that may be associated with AMD progression.
Follow-up
Patients with AMD should be followed at reasonable intervals to monitor for formation of CNVM (Table 3).29,30 Follow-up schedules can range from every four to 12 months, depending on the clinical picture, and any changes on home monitoring would warrant prompt examination.29,30 Depending on the clinical picture, follow-up visits may include fundus photography, Amsler grid, OCT, FAF, OCT-A or IVFA.29,30
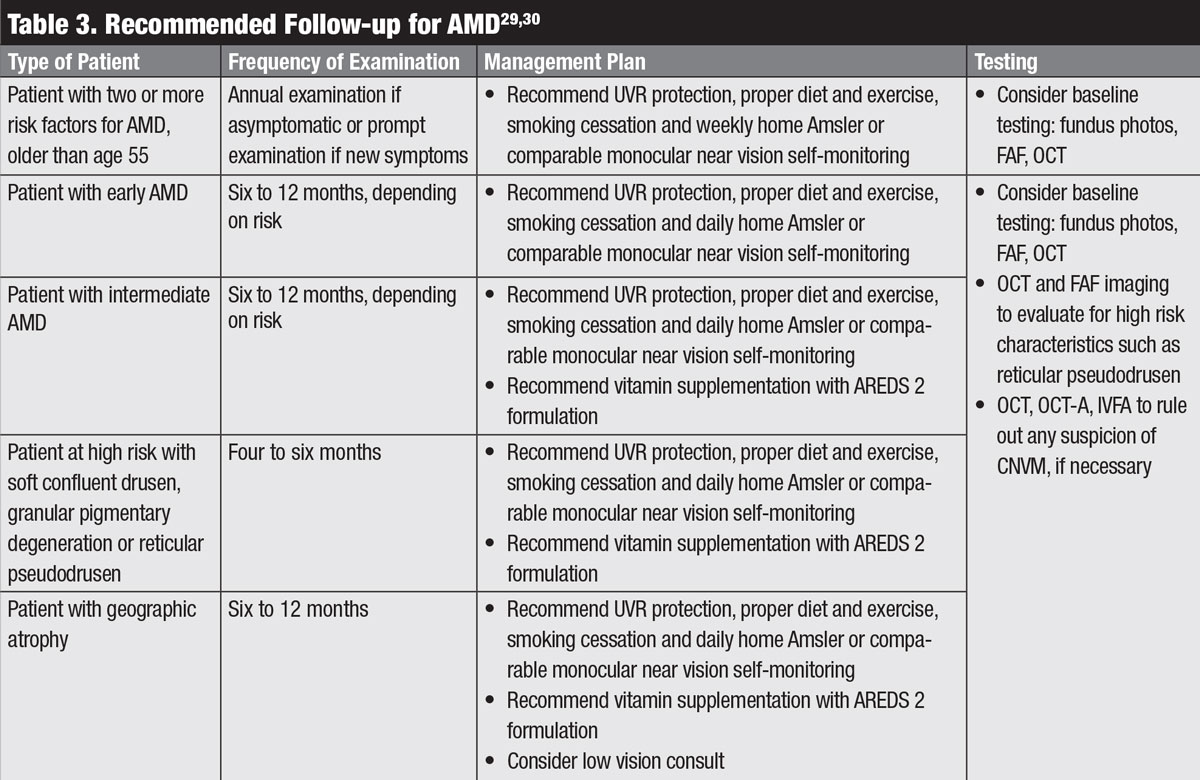 |
| Click table to enlarge. |
Patients’ visual limitations and complaints will worsen over time, and clinicians should address those issues optically or with additional devices such as magnifiers, telescopes, electronic visual aids and rehabilitative care to make the most of their remaining visual function. Consider a formal low vision evaluation or consultation if these services cannot be provided in your clinic. In addition, clinicians should address the patient’s legal driving status.
 |
| Click image to download this referral sheet. |
Communications
Patients with AMD often require referral and communication with a variety of healthcare providers, whether at the initial visit or at some point in their follow-up care. Clinicians should use a succinct, yet thorough, patient referral form when referring patients with AMD to other healthcare providers. The form should include basic patient information, your pertinent clinical findings, diagnosis, the services you are recommending, and any additional comments that could help the other provider best care for the patient.
The initial diagnosis of AMD carries significant responsibility for the diagnosing physician who must address patient concerns, manage progression risk factors, thoroughly evaluate the condition’s stage, determine follow up and communicate with other medical professionals. Balancing all of these during the initial visit is crucial to ensure the patient is prepared for the management road ahead.
Dr. Rafieetary is a consultative optometrist at the Charles Retina Institute in Germantown, Tenn.
Dr. Haynes is a consultative optometrist at the Charles Retina Institute and consulting faculty at Southern College of Optometry in Memphis, Tenn.
Dr. Attar is an assistant professor at the University of Mississippi Medical Center, Department of Ophthalmology.
|
1. Neely DC, Bray KJ, Huisingh CE, et al. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017;135(6):570. 2. Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137(3):486-95. 3. Velilla S, García-Medina JJ, García-Layana A, et al. Smoking and age-related macular degeneration: review and update. J Ophthalmol. 2013;2013:895147. 4. Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276(14):1141-46. 5. Sui G-Y, Liu G-C, Liu G-Y, et al. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013;97(4):389-94. 6. Carneiro Â, Andrade JP. Nutritional and lifestyle interventions for age-related macular degeneration: A review. Oxid Med Cell Longev. 2017;2017:1-13. 7. Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313-30. 8. Rim TH, Kim HK, Kim JW, et al. A Nationwide cohort study on the association between past physical activity and neovascular age-related macular degeneration in an East Asian population. JAMA Ophthalmol. 2018;136(2):132. 9. Gopinath B, Liew G, Flood VM, et al. Combined influence of poor health behaviours on the prevalence and 15-year incidence of age-related macular degeneration. Sci Rep. 2017;7(1):4359. 10. McGuinness MB, Le J, Mitchell P, et al. Physical activity and age-related macular degeneration: a systematic literature review and meta-analysis. Am J Ophthalmol. 2017;180:29-38. 11. Age-Related Eye Disease Study Research Group A-REDSR. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-36. 12. Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45(4):433-39. 13. Chew EY, Klein ML, Clemons TE, et al. Genetic testing in persons with AMD and the use of the AREDS supplements: to test or not to test? Ophthalmology. 2015;122(1):212-15. 14. ArcticDX. Macula Risk PGx DNA test. www.macularisk.com. Accessed June 4, 2019. 15. Awh CC, Lane A-M, Hawken S, et al. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013;120(11):2317-23. 16. Chew EY, Klein ML, Clemons TE, et al. No clinically significant association between CFH and ARMS2 genotypes and response to nutritional supplements. Ophthalmology. 2014;121(11):2173-80. 17. Assel MJ, Li F, Wang Y, Allen AS, Baggerly KA, Vickers AJ. Genetic polymorphisms of CFH and ARMS2 do not predict response to antioxidants and zinc in patients with age-related macular degeneration: independent statistical evaluations of data from the Age-Related Eye Disease Study. Ophthalmology. 2018;125(3):391-97. 18. Owsley C, McGwin G, Clark ME, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early AMD. Ophthalmology. 2016;123(2):344-51. 19. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-51. 20. Goldenberg D, Shahar J, Loewenstein A, Goldstein M. Diameters of retinal blood vessels in a healthy cohort as measured by spectral domain optical coherence tomography. Retina. 2013;33(9):1888-94. 21. Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration. Arch Ophthalmol. 2005;123(11):1570. 22. Lee JY, Lee DH, Lee JY, Yoon YH. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Opthalmol Vis Sci. 2013;54(12):7812. 23. Joachim N, Mitchell P, Rochtchina E, et al. Incidence and progression of reticular drusen in AMD: findings from an older Australian cohort. Ophthalmology. 2014;121(4):917-25. 24. Gil JQ, Marques JP, Hogg R, et al. Clinical features and long-term progression of reticular pseudodrusen in age-related macular degeneration: findings from a multicenter cohort. Eye (Lond). 2017;31(3):364-71. 25. Talks SJ, Aftab AM, Ashfaq I, Soomro T. The role of new imaging methods in managing age-related macular degeneration. Asia-Pacific J Ophthalmol. 2017;6(6):498-507. 26. Ly A, Nivison-Smith L, Assaad N, Kalloniatis M. Fundus autofluorescence in age-related macular degeneration. Optom Vis Sci. 2017;94(2):246-59. 27. Gong J, Yu S, Gong Y, et al. The diagnostic accuracy of optical coherence tomography angiography for neovascular age-related macular degeneration: a comparison with fundus fluorescein angiography. J Ophthalmol. 2016;2016:7521478. 28. Ying GS, Huang J, Maguire MG, et a. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular AMD. Ophthalmology. 2013;120(1):122-29. 29. Zajac-Pytrus HM, Pilecka A, Turno-Krecicka A. The dry form of age related macular (AMD) degeneration: the current concepts of pathogenesis and prospects for treatment. Adv Clin Exp Med. 2015;24(6):1099-1104. 30. American Optometric Association. Optometric clinical practice guideline. Care of the patient with age-related macular degeneration. 2004. www.aoa.org/documents/optometrists/CPG-6.pdf. Accessed May 24, 2019. |

