24th Annual Glaucoma ReportFollow the links below to read other articles from annual update on glaucoma: A Guide to Applying IOP-lowering Drugs Comanaging Invasive Glaucoma Surgeries Glaucoma: Lifestyles of the Antioxidant Rich and Famous (Earn 2 CE Credits) |
Traditionally, glaucoma management consisted of three choices, with varying levels of efficacy: eye drops, laser trabeculoplasty and, for progressing and severe glaucoma, bleb-forming surgery. However, side effects, ocular surface toxicity, out-of-pocket costs and patient noncompliance pose problems with long-term topical options.1 Risks associated with filtering surgery, such as infection, hypotony, inflammation and bleb-related complications, may be problematic as well. Even selective laser trabeculoplasty, an excellent treatment option for many patients, has diminished efficacy over time.2
Luckily, patients with mild to moderate glaucoma have a new management option with the advent of minimally invasive glaucoma surgery (MIGS). These procedures have an ab interno approach, cause minimal trauma to the sclera and little to no scleral dissection, minimal or no conjunctival manipulation, are modestly effective and have good safety profile and rapid recovery.3
MIGS now consist of at least 15 different techniques, making it difficult to stay current on the advancements and efficacies of each procedure. Here, we review the current MIGS and offer guidance on patient selection and procedure recommendations.
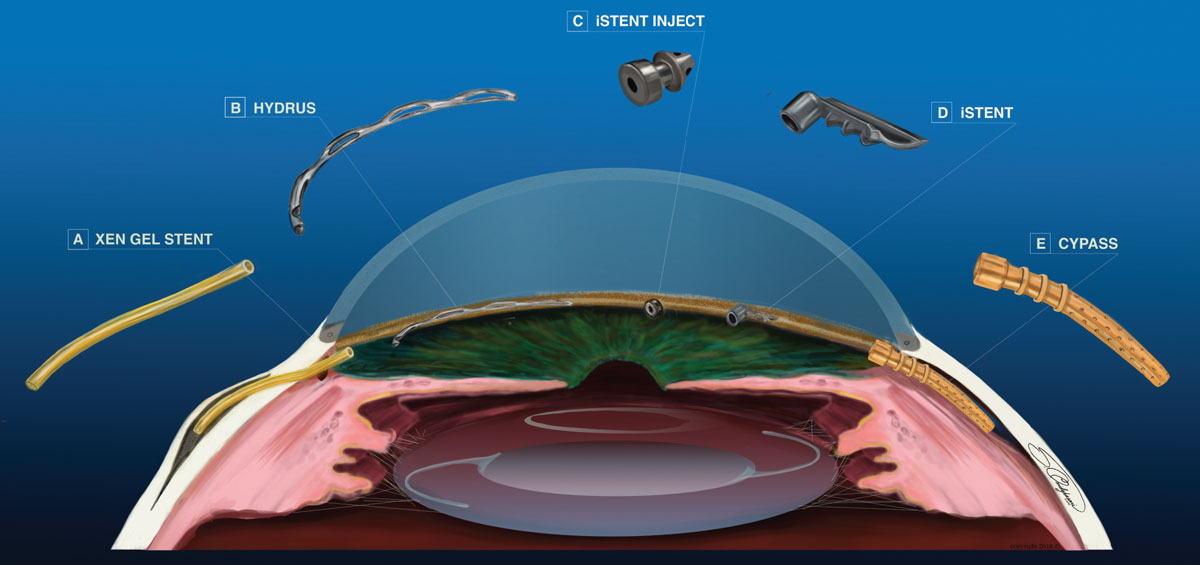 |
| Fig. 1. Each of these MIGS procedures can reduce IOP, but in distinctly different ways. Click image to enlarge. |
Trabecular Meshwork Bypass
Several devices use this technique:
iStent (Glaukos). This L-shaped microstent was the first FDA-approved MIGS device in 2012 (Figure 1d). The surgeon inserts the lumen into Schlemm’s canal, with the neck extending into the anterior chamber, to create a direct connection from the anterior chamber to Schlemm’s canal. By bypassing the trabecular meshwork (TM), the stent is designed to increase aqueous outflow.3
iStent is approved for patients in conjunction with cataract surgery, with stable, mild-to-moderate primary open-angle glaucoma (POAG) on one to three pressure-lowering medications, and with a target intraocular pressure (IOP) of mid teens or higher. One study shows that patients who underwent cataract extraction combined with iStent had an 8% reduction in IOP and 1.3 fewer hypotensive medications after a two-year follow-up.4 The safety profile for iStent combined with cataract extraction was equal to that of cataract extraction alone.4 Complications include stent malposition and obstruction. To date, no adverse events have been reported in association with iStent complications.
Many factors, including proper position of the stent and its proximity to collector channels, may affect the device’s efficacy.5 Additionally, the post-op IOP will not fall below the episcleral venous pressure, which may be elevated in some POAG patients.6 The maximum IOP lowering effect will be achieved six to eight weeks after implantation.
iStent inject (Glaukos). This device’s injector houses two iStents and is designed for easier injection and to reduce both surgery time and the risk of infection (Figure 1c). The appearance of the second-generation stent is similar to that of a punctal plug, with a linear, not L, shape. The method of action for iStent inject is identical to that of the original iStent. These microstents are inserted in the nasal angle two clock hours apart in a procedure combined with cataract surgery. A study found a 15% IOP reduction after two years and 18.9% reduction after five years, with 1.00 and 0.21 fewer medications, respectively.7 iStent inject, with a similar safety profile and complications as the original, was approved by the FDA in June.
Hydrus microstent (Ivantis). This is an 8mm device used to improve aqueous outflow in three ways (Figure 1b). The Hydrus provides direct connection between the anterior chamber and Schlemm’s canal. Due to its length, it creates a three clock-hour scaffolding of the canal. Additionally, three windows on the anterior face of the device stretch the TM and increase aqueous outflow.
The Hydrus is projected for FDA approved in late 2018 for use in conjunction with cataract surgery. The stent is preloaded in an inserting device and is placed through the same clear cornea incision used for cataract surgery. Ideal patients would be those with similar glaucoma severity and treatment profile as iStent candidates, with the exception that, for proper device placement, Hydrus candidates should have at least moderately pigmented TM for easier anatomical location.
One study found average reduction in IOP was 12.7% with patients using 1.5 fewer medications two years after Hydrus combined with cataract surgery.8 The most common complication found in this study was peripheral anterior synechia near the inlet segment of the device, which did not affect IOP reduction.8
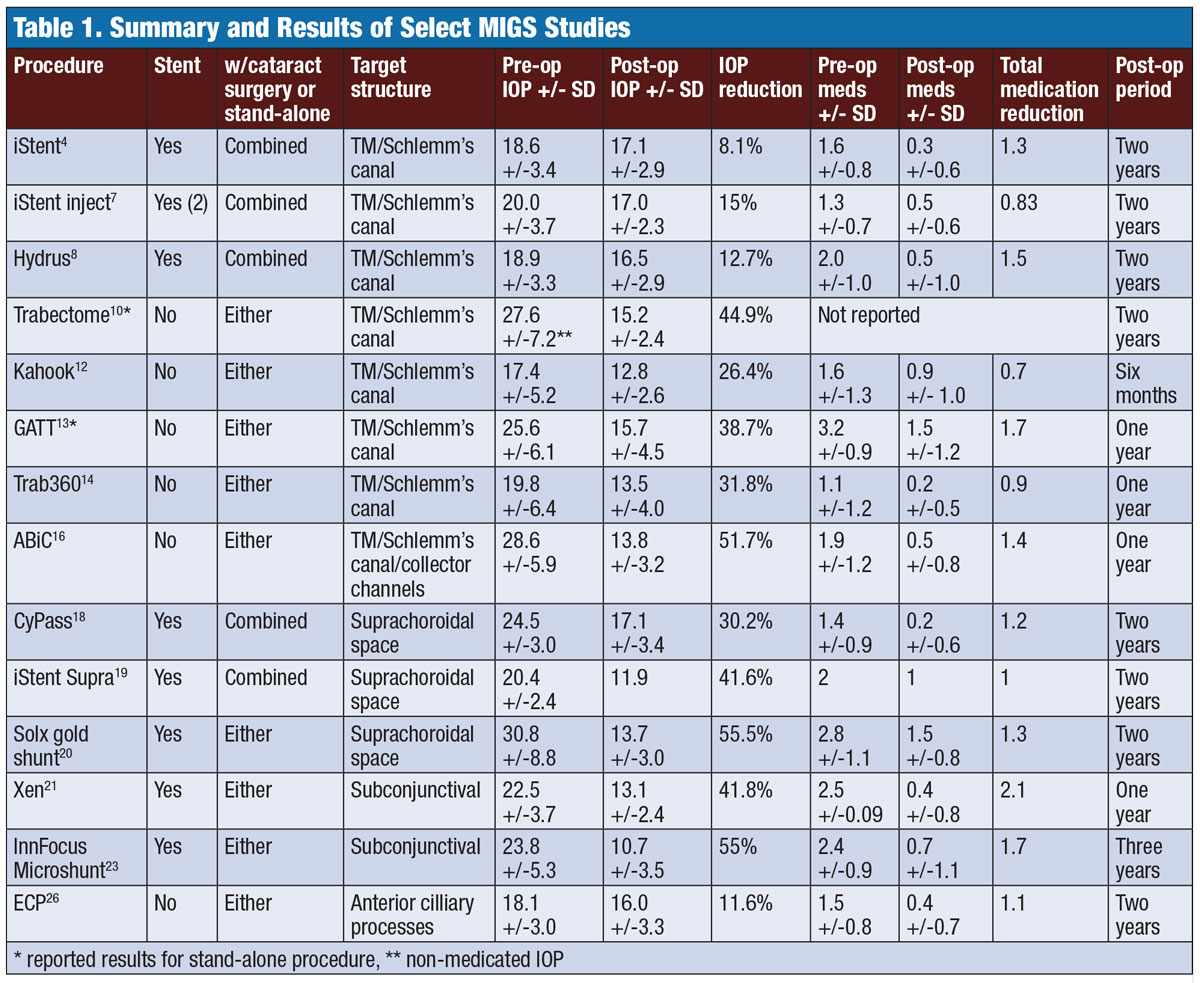 |
| Table 1. Summary and Results of Select MIGS Studies. Click image to enlarge. |
Trabeculotomy
The efficacy of all delineated TM/Schlemm’s canal procedures depends on the integrity of the collector channels downstream, as damage or occlusion limits the effect. The following TM/Schlemm’s canal procedures are better suited for younger patients who are more likely to have an intact collector channel system:9
Trabectome (NeoMedix). This electrocautery device is used to perform a partial trabeculotomy (Figure 2c). The surgeon inserts the device through a temporal clear corneal incision and directs it to the nasal portion of the angle. The TM and interior wall of Schlemm’s canal are removed via cauterization anywhere from 90° to 180°. Aspiration and irrigation ports on the tip of the device maintain homeostasis during the procedure. Trabectome is approved as a stand-alone procedure or in combination with cataract surgery. Contraindications include narrow angle (less than 20°), increased episcleral venous pressure, limited neck mobility, neovascularization of the angle or iris and corneal opacity obstructing the view of the angle.
Complications include blood reflux into the anterior chamber with up to 20% hyphema and partial goniosynechiae. These did not cause a significant IOP increase or failure of the procedure.10 Two studies show 23% and 44% IOP reduction two years after trabectome.10,11
Kahook Dual Blade (New World Medical). This single-use knife is designed to remove the TM and Schlemm’s canal internal wall without damage to adjacent tissues (Figure 2d). The surgeon inserts the knife through a temporal clear corneal incision during cataract surgery or in a stand-alone procedure. A section of nasal TM (up to 180°) is excised and removed with forceps or aspiration. Studies show the mean baseline IOP decreased by 26.4% with an average of 0.7 fewer medications six months post-procedure.12
An advantage of this procedure over Trabectome is the lack of thermal damage to the angle and reduced risk of peripheral anterior synechia postoperatively. As with all trabeculotomies, hyphema is the most common complication and typically resolves within one month.
Gonioscopy-assisted transluminal trabeculotomy (GATT). During this 360° trabeculotomy, the surgeon creates a temporal clear corneal incision and inserts forceps (Figure 2a). A suture or microcatheter is inserted through a separate clear corneal incision. A small goniotomy is created nasally in the TM, and the suture or microcatheter is threaded through the circumference of Schlemm’s canal. The suture is then pulled through to unroof the TM.
The procedure can be performed during cataract surgery or alone in a phakic or pseudophakic eye. Researchers report an average of 39.8% IOP reduction and 1.1 fewer medications in postoperative GATT patients with POAG.13 The most common complication is postoperative hyphema, which typically self resolves within a month. Advantages include its low cost, especially with suture material, and the full 360° treatment of the angle.
Trab360 (Sight Sciences). This 360° trabeculotomy is performed through one clear corneal incision temporally, where the Trab360 device is inserted into the anterior chamber (Figure 2b). The tip pierces the TM nasally and a probe contained within the device is advanced 180° into Schlemm’s canal; the surgeon then extracts the device from the eye, cutting through the TM and creating a 180° trabeculotomy. The surgeon can retract the probe and use the device to treat the remaining 180° of TM. Trab360 can be performed in conjunction with cataract surgery or as a stand-alone procedure. Initial results show an average IOP reduction of 31.8% and patients required 0.9 fewer meds.14
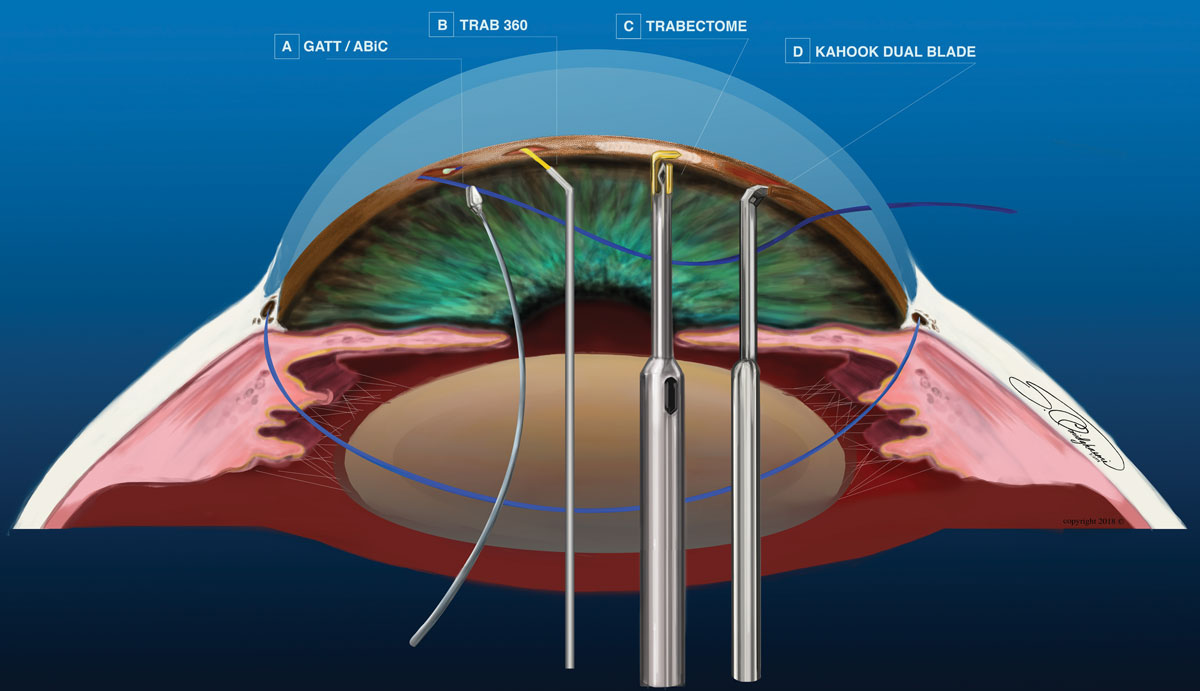 |
| Fig. 2. Rather than use an implanted device, many MIGS use a blade, laser or sutures to complete the procedure. Click image to enlarge. |
Natural Outflow Enhancement
A slight variation on traditional ab externo canaloplasty, ab interno canaloplasty (ABiC) (iTrack, Ellex) treats all aqueous outflow structures (Figure 2a). As with GATT, a microcatheter device is advanced through Schlemm’s canal 360°. Instead of pulling the microcatheter to cut the TM, the surgeon extracts it as viscoelastic is gently injected into Schlemm’s canal. This viscodilation breaks adhesions within Schlemm’s canal, stretches the trabecular plates and creates microperforations within the inner wall. It also improves outflow by irrigating collector channel blockages.15
Multiple MIGSIt is becoming increasingly popular for surgeons to perform multiple MIGS in the same surgery to combine different methods of action or target tissue to maximize IOP lowering effect. Two common combination procedures include the Omni device (Sight Sciences), which is a combination of Visco360 and Trab360 procedures, and ICE, which involves iStent implantation in conjunction with cataract extraction and ECP. |
With ABiC, all structures of the aqueous pathway remain intact. Currently, it is the only MIGS that addresses collector channel blockages. ABiC can be a stand-alone procedure or done in conjunction with cataract surgery in primary and secondary glaucoma. The procedure is not suitable for angle closure, neovascular or traumatic glaucoma. Results of ABiC alone one year after surgery show average IOP fell by 19.3%, and patients used 1.4 fewer medications. ABiC combined with cataract surgery can provide an average IOP reduction of 51.7% and 1.4 fewer medications after one year.16
In the same fashion as ABiC, Visco360 (Sight Sciences) is designed to viscodilate Schlemm’s canal and collector channels using the Trab360 device to perform the surgery.
Suprachoroid
The CyPass (Alcon) is an ab interno suprachoriodal shunt made of brown polymide material (Figure 1e). It has a 0.3mm lumen, is 6.35mm in length and comes preloaded in a curved inserter. The surgeon inserts the device through a temporal clear corneal incision into the nasal angle posterior to the scleral spur, with the distal portion of the device embedded in the suprachoroidal space. The shunt diverts aqueous from the anterior chamber into the suprachoroidal space using the uveoscleral outflow system. Aqueous not only flows though the lumen of the stent, but also through fenestrations in the distal portion of the device.17 The COMPASS trial two-year outcomes show mean IOP was reduced by 30.2% and 1.2 fewer medications.18 Infrequent and largely transient adverse events included reduced vision by more than two lines, visual field progression, iritis and corneal edema. Stent obstruction occurred in 2.1% of patients due to focal peripheral anterior synechia.18 CyPass is FDA-approved for use in open angle glaucoma with cataract surgery.
iStent Supra (Glaukos). This suprachoroidal shunt is comprised of polyethersulfone and titanium. The placement and mechanism of action are identical to the CyPass stent. iStent Supra is currently available in Europe and Canada and is in US clinical trials for insertion combined with cataract surgery.19
Solx gold shunt (Solx). Made of 24-carat gold, this 5.5mm by 3.2mm flat stent is inserted ab externally through a scleral incision in any quadrant of the eye. The anterior portion of the stent is placed 1mm into the anterior chamber with the posterior end of the stent in the suprachoroidal space. Created for use in patients with refractory glaucoma, this device is approved for use in Europe and Canada and is currently in clinical trials for FDA approval as a stand-alone procedure or combined with cataract surgery.20
Subconjunctiva
The Xen gel stent (Allergan) is a gelatin and glutaraldehyde 6mm tube, which is preloaded in a disposable injector (Figure 1a). The surgeon inserts the injector through a clear cornea incision and tunnels through the sclera at or anterior to Schlemm’s canal to deploy the distal portion of the stent within the subconjunctival space. This creates a pathway for aqueous to flow from the anterior chamber to the subconjunctival space, forming a bleb. The procedure may include subconjunctival injection of mitomycin C to prevent scar formation and bleb failure.21
Xen is FDA approved for refractory glaucoma. It is useful in patients with primary, pseudoexfoliative or pigmentary glaucoma with open angles. It is reserved for patients who have progressive disease despite maximum medical treatment, and who failed previous surgical interventions. A recent study of 41 eyes with the Xen implant combined with cataract surgery shows an average IOP reduction of 41.8% and 2.1 fewer medications post-procedure.22 Complications were rare in this study, and included one case of each of the following: bleb requiring needling, device explant, device migration, device obstruction, transient hypotony and transient choroidal detachment.22
The InnFocus MicroShunt (InnFocus, Santen) is a 8.5mm long implant made of poly(styrene-block-isobutylene-block-styrene). The surgeon inserts this device into the anterior chamber through an ab externo approach, creating a bleb in the subconjunctival space. During the procedure, mitomycin C is applied to the area of the intended bleb to reduce the risk of scar formation and bleb failure. The procedure may be performed in combination with cataract surgery or alone, and is approved for use in Europe. It is currently in clinical trials for approval in the United States.23
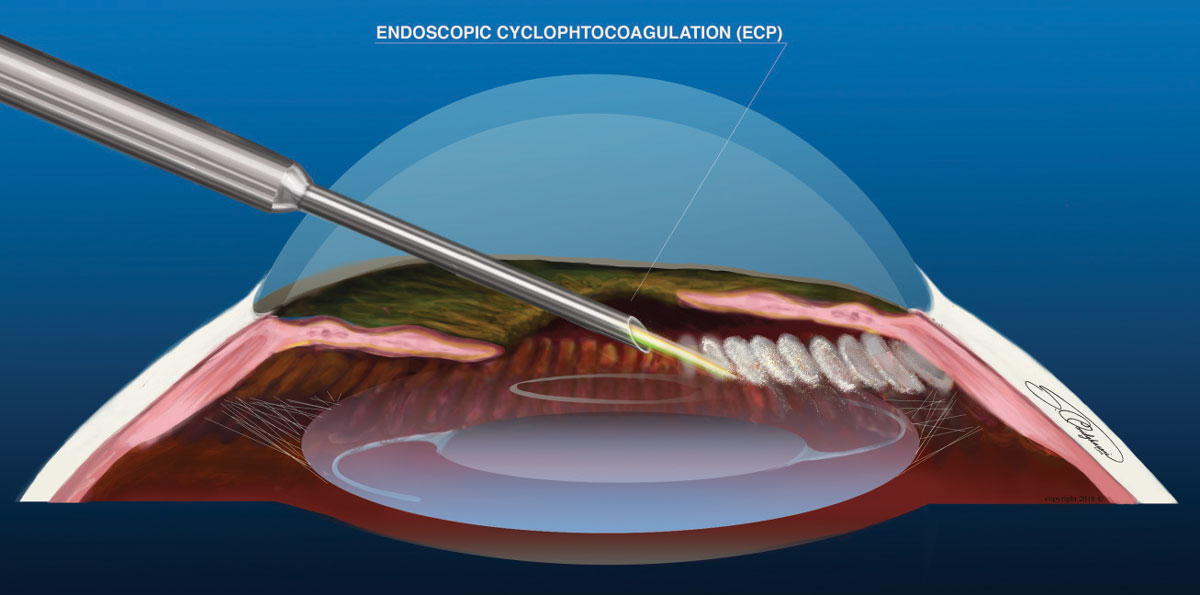 |
| Fig. 3. ECP is the only MIGS procedure that decreases aqueous production. Click image to enlarge. |
Cyclophotocoagulation
The endoscopic cyclophotocoagulation (ECP, Beaver-Visitec International) probe is a reusable device consisting of a laser, aiming beam, light source and field view endoscope. The surgeon inserts the endolaser device through a clear corneal incision, directs the probe posterior to the iris and treats the anterior cilliary processes with photocoagulation (Figure 3). Roughly 240° to 300° of cilliary body processes can be treated with a single incision; a full 360° treatment requires a second incision. Research shows a direct correlation between the amount of cilliary processes treated and the effectiveness of the procedure.24 It can be used in open- or closed-angle glaucoma, as an initial treatment option for mild-to-moderate glaucoma, or for advanced glaucoma for which previous management has failed.25 One study shows a mean IOP reduction of 11.6% and medication reduction by 1.1 for patients who had ECP with cataract surgery.26
While a useful procedure, it comes with possible complications, including: chronic inflammation, cystoid macula edema, cataracts, hypotony and phthisis bulbi. ECP may be performed during cataract surgery or alone. Due to the risk of cataract development, ECP it is better suited for phakic patients or in combination with cataract surgery.
A similar procedure, micropulse transscleral cyclophotocoagulation (Cyclo G6/MicroPulse P3, Iridex), staggers laser energy output into pulses to minimize iatrogenic damage. An investigational technique called ultrasound-mediated cyclomodification uses ultrasonic energy to produce controlled thermocoagulation without deleterious effects to surrounding tissue.26
Patient Selection
While the final decision is often in the surgeon’s hands, optometrists are key players in comanagement. ODs will be faced with the first patient encounter and must consider many education factors, including the fact that cataract surgery alone can lower IOP.27 For those with cataracts and mild-to-moderate glaucoma who are stable on one medication, iStent combined with cataract extraction would be a good option as well.27 Referral to a skilled cataract surgeon with iStent experience or a glaucoma specialist would be appropriate.
Glaucoma patients on multiple medications with a goal IOP of mid teens or higher and visually significant cataracts would benefit from iStent inject, Hydrus, Trabectome, Kahook, GATT, Trab 360, ABiC, CyPass or ECP combined with cataract surgery rather than cataract surgery alone. Pseudophakic patients whose goal IOP is not met with medical management or are non-compliant with medications could be considered for Trabectome, Kahook, GATT, Trab 360, ABiC or ECP. Xen gel stent is best suited for patients with refractory glaucoma.
Patients with a target IOP of 12mm Hg or lower will achieve minimal benefit from a TM/Schlemm’s procedure and would be better served with CyPass combined with cataract surgery or Xen stent.28
A MIGS referral should include the following, whenever possible:
- Maximum IOP and IOP on current treatment.
- Current glaucoma medications and any that were ineffective or not tolerated.
- Baseline and current visual field and any fields demonstrating progression.
- Gonioscopic assessment of the angle structures.
- Baseline and current optic nerve OCT and glaucoma progression analysis.
- Eye surgery/injury history.
Providing this information in your referral will eliminate repeat testing and inform the surgeon of the patient’s disease process, allowing the surgeon to choose the best MIGS procedure for the patient.
It is an exciting time to treat glaucoma, as patients now have a middle road to travel for management. A basic understanding of the available MIGS procedures and their individual niches in glaucoma management can help you educate patients properly, refer with confidence, and ultimately reduce the need for medication and thus the costs and inconvenience to the patient.
Dr. Caywood is a staff optometrist at the Oklahoma City VAMC.
Dr. Omidghaemi is a talented artist and a staff optometrist at the Oklahoma City VAMC.
1. Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015 May;9:785-93. 2. Leahy KE, White AJ. Selective laser trabeculoplasty: current perspectives. Clin Ophthalmol. 2015 May 11;9:833-41. 3. Saccà SC, Gandolfi S, Bagnis A, et al. The outflow pathway: a tissue with morphological and functional unity. J Cell Physiol. 2016;231(9):1876-93. 4. Craven ER, Katz LJ, Wells JM, et al. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339-45. 5. Yuan F, Schieber AT, Camras LJ, et al. Mathematical modeling of outflow facility increase with trabecular meshwork bypass and schlemm canal dilation. J Glaucoma. 2016;25(4):355-64. 6. Bostan C, Harasymowycz P. Episcleral venous outflow: a potential outcome marker for iStent surgery. J Glaucoma. 2017;26(12):1114-9. 7. Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Glaukos iStent inject trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1056573. 8. Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122(7):1283-93. 9. Fellman RL, Grover DS. Episcleral venous fluid wave: intraoperative evidence for patency of the conventional outflow system. J Glaucoma. 2014;23(6):347-50. 10. Minckler D, Baerveldt G, Ramirez MA, et al. Clinical results with the Trabectome, a novel surgical device for treatment of open-angle glaucoma. Trans Am Ophthalmol Soc. 2006;104:40-50. 11. Kaplowitz K, Schuman JS, Loewen NA. Techniques and outcomes of minimally invasive trabecular ablation and bypass surgery. Br J Ophthalmol. 2014 May;98(5):579-85. 12. Greenwood MD, Seibold LK, Radcliffe NM, et al. Goniotomy with a single-use dual blade: Short-term results. J Cataract Refract Surg. 2017;43(9):1197-1201. 13. Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855-61. 14. Sarkisian SR, Allen E, Ding K, et al. New way for ab interno trabeculotomy: initial results. Poster presented at ASCRS/ASOA Symposium & Congress, April 17, 2015; San Diego. 15. Khaimi MA. Canaloplasty: A minimally invasive and maximally effective glaucoma treatment. J Ophthalmol. 2015;2015:485065. 16. Khaimi M. Twelve-month follow-up of ab interno canaloplasty as a standalone treatment and in adjunct to cataract surgery for the treatment of primary open-angle glaucoma. Poster presented at The 27th Annual AGS Meeting, March 9, 2017; Coronado, CA. 17. Hoeh H, Vold SD, Ahmed IK, et al. Initial clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary microstent. J Glaucoma. 2016;25(1):106-12. 18. Vold S, Ahmed II, Craven ER, et al.; CyPass Study Group. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123(10):2103-12. 19. Myers JS, Katz LJ. Results of suprachoroidal stent and topical travoprost for reduction of IOP and medication in open-angle glaucoma. Paper presented at: The 24th Annual AGS Meeting; February 27, 2014; Washington, DC. 20. Figus M, Lazzeri S, Fogagnolo P, Iester M, Martinelli P, Nardi M. Supraciliary shunt in refractory glaucoma. Br J Ophthalmol. 2011 Nov;95(11):1537-41. doi: 10.1136/bjophthalmol-2011-300308. Epub 2011 Aug 26. 21. Chaudhary A, Salinas L, Guidotti J, et al. XEN gel implant: a new surgical approach in glaucoma. Expert Rev Med Devices. 2018;15(1):47-59. 22. De Gregorio A, Pedrotti E, Russo L, Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. May 29, 2017. [Epub ahead of print]. 23. Batlle JF, Fantes F, Riss I, Pinchuk L, Alburquerque R, Kato YP, Arrieta E, Peralta AC, Palmberg P, Parrish RK 2nd, Weber BA, Parel JM. Three-Year Follow-up of a Novel Aqueous Humor MicroShunt. J Glaucoma. 2016 Feb;25(2) 24. Kahook MY, Lathrop KL, Noecker RJ. One-site versus two-site endoscopic cyclophotocoagulation. J Glaucoma. 2007;16(6):527-30. 25. Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25-36. 26. Aptel F, Charrel T, Lafon C, et al. Miniaturized high-intensity focused ultrasound device in patients with glaucoma: a clinical pilot study. Invest Ophthalmol Vis Sci.2011;52(12):8747-8753. 27. Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40(8):1313-21. 28. Rabin RL, Rabin AR, Zhang AD, et al. Co-management of cataract and glaucoma in the era of minimally invasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29(1):88-95. |

