With the introduction of the first eye drop for presbyopia, optometrists have another tool in their arsenal to alleviate this vexing condition. As with any new therapeutic approach, it is important to understand the benefits and risks, as well as the implications for clinical practice.
“It is an exciting time in eye care,” notes Melissa Barnett, OD, of the University of California, Davis Eye Center in Sacramento and Davis, CA. “With the approval of Vuity (pilocarpine hydrochloride, Allergan), we now have a whole new class of medications at our disposal. And, not only that, we also have a new practice modality and the opportunity to offer our patients more than glasses, contact lenses and surgical options.”
Given the prevalence of the condition—there are currently 128 million presbyopes in the United States, and that number will only continue to grow—the implications of a pharmaceutical option are significant, according to Dr. Barnett. “This is just the beginning,” she says, while noting there are a number of products in development right now. “Therefore, it is important that doctors of optometry consider the impact that therapeutics like Vuity can have for patients and their practices.”
As this new approach is explored in clinical practice, optometrists are tasked with educating patients and helping them determine if this is the right option for their needs and lifestyle. This involves considering the pros and cons of the drug, as well as how it compares to the current standard of care. Corrective lenses may be bothersome, but they set various benchmarks for performance in the minds of the public and the doctors who serve them. A medical alternative that fails to provide comparable results must offer substantial advantages in return for the trade-off to be worthwhile.
 |
|
Eyedrop therapy now challenges the gold standard of corrective lens options for presbyopia. ODs need to understand the pros and cons of each and be candid when discussing them. Photos: Getty Images. Click image to enlarge. |
Pilocarpine in Practice
Vuity uses the familiar pupil-constricting properties of pilocarpine (at a 1.25% concentration) to temporarily sharpen near vision. In an attempt to reduce stinging upon instillation, the drug is formulated in such a way that it rapidly adjusts to the natural pH of the tear film.1
It received FDA approval in October 2021 for patients with presbyopia based on findings from two Phase III clinical trials that evaluated the efficacy, safety and tolerability of Vuity in presbyopic patients between the ages of 40 and 55. The treatment showed a statistically significant improvement when compared to placebo. The primary endpoint was the ability to achieve three lines of near vision improvement under mesopic conditions without losing more than one line of distance vision at day 30, hour three.1
In terms of side effects, the study authors reported that the most common non-serious adverse events were headache and eye redness.2-4 The research also showed that the effect of the drug was seen as early as 15 minutes, peaked after one hour and then declined over time, lasting for about six hours.
Considerations For the OD
Now that a pharmaceutical option is available for presbyopia, optometrists should be ready to have discussions with their patients that include the benefits and potential adverse events, as well as realistic expectations on performance and cost.
But first, they must identify the ideal candidate for Vuity. “I would have some discussion with any patients experiencing early symptoms of presbyopia—generally after age 40—and those who are in their mid-50s,” says Joseph Shovlin, OD, of Northeastern Eye Institute in Scranton, PA, who notes that it’s worth remembering contact lens patients may experience near problems earlier than they might with spectacle correction.
To date, Dr. Shovlin has prescribed this treatment to a few patients but cannot yet comment on its durability. “Some of my patients have expressed a dimming of vision effect initially, and most have continued use with less of a dimming effect over time,” he explains, while suggesting prescribers stick to the recommended cut-off of 55 years old, since most patients will experience at least some early lens changes at 55 and older.
“Those of us who remember prescribing pilocarpine for glaucoma—when we had just this class along with a beta-blocker—recall the undesirable side effects with patients who had significant cataracts and those who experienced the common side effects of cholinergic muscarinic agonists, namely conjunctival injection and headaches,” Dr. Shovlin adds.
Since Vuity’s approval, Dr. Barnett has seen significant excitement among her patients, including those who have brought up the treatment themselves. The feedback she has received so far has been positive. “My patients have been very happy with their results and are thrilled to have an option to correct their vision that works. Very few have reported headaches, and if a headache is noticed, it is typically on the first day,” she notes.
“We have found that using the drops every morning for a week helps with adaptation,” Dr. Barnett adds. “I also ask very detailed questions at the initial examination and in my follow-up to better understand how this treatment fits into my patient’s lifestyle.”
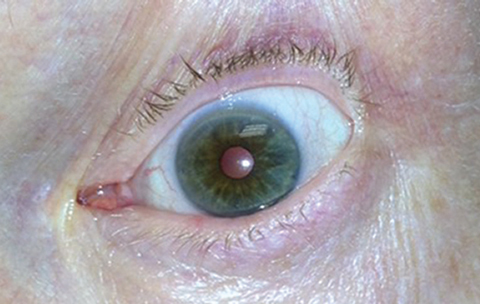 |
|
Pupil constriction following (therapeutic) pilocarpine use. ODs are familiar with previous incarnations of the drug after decades of medical use prior to its FDA approval for presbyopia. Photo: Michael Trottini, OD, and Michael DelGiodice, OD. Click image to enlarge. |
Justin Bazan, OD, of Park Slope Eye in Brooklyn, has also found early success with the drug. “It has been a welcomed addition to my optometric toolbox,” he notes. “For my pre-presbyopes, I give them the heads up and get them thinking about it. It often gets a ‘wow’ and reminds them that I’m a doc who is passionate about their care and who stays current in the industry.”
Other optometrists have expressed frustration and seen variable results. The main issues include the aforementioned dimming of vision, particularly while indoors, and inconsistent duration of effect. Some noted that the drug stopped working within a few hours, while it lasted up to seven hours in other patients.
The unpredictable duration could be a deterrent for some patients, especially those whose workday is eight hours or longer. This may mean that contact lenses or glasses might still make the most sense as a primary corrective modality, at least during the work week. Given Vuity’s newness as of this writing, the profession has yet to develop even anecdotal impressions of the implications for usage beyond the FDA recommended dosage of one drop in each eye once daily.
Still, the general consensus appears to be that Vuity should be explored and embraced as a new avenue of treatment for some well-chosen and appropriately educated patients. Prepping the patient is key and, as always, it is important to share all available options while being mindful to thoroughly explain the pros and cons of each.
For Paymaun Asnaashari, OD, of Helmus Optometry in Sacramento, CA, the patients he has successfully prescribed Vuity for are the ones who come to their exam already interested in hearing more about this approach. People who are learning about it for the first time from him are harder to convince. “I have brought this treatment up to multiple patients, especially the presbyopes who are starting to lose their ability to read, and, so far, many are hesitant to try it,” he explains.
According to Dr. Asnaashari, one point of resistance for his patients has been the cost. Currently, Vuity is not covered by insurance and, at $80 per 30-day supply, can be a significant out-of-pocket expense. If patients and practitioners begin to develop off-label protocols that involve more frequent dosing than QD to extend the drug’s effect, the cost proposition obviously worsens as a consequence. Being upfront with your patients about the cost and other potential cons is key, he suggests.
While Dr. Shovlin doesn’t think cost will be a significant barrier to use overall, he does have some concerns that the price tag could prompt individuals to attempt to obtain pilocarpine 1%. “I have heard that already some areas of the country have limited stock of generic pilocarpine for other uses,” he says. “It’s important to remind our patients and colleagues that this is the same drug but not the same compound, since Vuity has a unique vehicle and formulation.”
Another important topic is correct usage and timing. Dr. Shovlin says patients should exercise caution early on while driving until they’ve gauged their comfort. Dr. Barnett adds that contact lens wearers should be advised to instill the drops first, then apply their contact lenses 15 minutes later.
Additionally, make time to have a discussion with your patients prior to anticipated dilation so they can stop using the drop a few days prior, according to Dr. Shovlin, who acknowledges that this could be a challenge for patients who present with acute symptoms of retinal pathology, such as flashes, floaters, specks and cobwebs.
“I do have some reservations in patients who might use something like this for years prior to cataract surgery and may not get adequate dilation for their cataract removal,” notes Dr. Shovlin. “If prescribing recommendations continue to highlight age 55 as the cutoff, this shouldn’t pose a major problem.”
While serious adverse events are exceedingly rare, ODs should remain aware of issues such as iris cysts, angle widening resulting in closure, accommodative spasm and retinal detachment in myopes, Dr. Shovlin advises.
As with any therapeutic option, it is up to the optometrist to inform their patients of the risks vs. benefits, as well as to help them determine if this treatment makes sense for their individual needs. “The drug is readily available and easy to administer with fast onset and sufficient duration. Patients will know pretty early on whether this drop makes sense for them,” says Dr. Shovlin. “Overall, the response has been favorable for those who are properly screened and have had an adequate education on how to best use the drop.”
One Optometrist’s Cautionary TaleWhen she first received an inquiry about Vuity, Susan Caul, OD, who practices in Redwood City, CA, decided to try the drug herself before prescribing it to any of her patients. On the morning of February 6, she instilled one drop in each eye. About 10 to 15 minutes later, she recalls her right eye starting to develop a central pixelated-like spot that progressed to a brown dusty spot and then a central blur. She reached out to two retina specialists she refers to who advised her to wait it out, as the side effects would most likely resolve when the drop wore off. Six to eight hours later, the scotoma had shrunk but not resolved. She went to her office the next morning for a macular OCT, which showed a subfoveal outer retinal disruption. Her corrected acuity had dropped from 20/20 to 20/25, and she had a small central scotoma that obscured about half of the number or letter that she was looking at.
Two weeks later, the scotoma had still not fully resolved. Follow-up OCT every few days showed the disruption to be shifting slightly in shape, but her visual disturbance was unchanged. “I have not had a posterior vitreous detachment in this eye, but I had one in my fellow eye in 2019,” she explains. “I did a literature search and found a similar case with administration of pilocarpine 2%. The mechanism was presumed to be vitreofoveal traction due to a forward displacement of the lens.”1 Regarding her near vision status post-Vuity, Dr. Caul says she still needed to use her reading glasses to see print. “It was hard to tell if there was any improvement in my near visual acuity, and my visual disturbance clouded any near visual acuity benefit that may have occurred,” she notes. “I also experienced a sort of ‘jiggling’ in my vision as though I had nystagmus in the first 30 minutes after installation only when I used my computer.” Based on her personal experience, she says, “Although pilocarpine was used for years as a glaucoma treatment and this drop was approved by the FDA for presbyopia, there are potential side effects that could possibly be serious. Anyone prescribing this needs to be aware of potential risks and make sure the patient is informed.”
|
Implications For Optometric Practice
In addition to offering presbyopic patients another option, Vuity can also be a source of added value for optometrists and their practice. “A doctor who presents a comprehensive range of options is recognized and appreciated by their patients,” Dr. Bazan notes. “It helps solidify the doctor as an expert in solving the visual needs of a patient and strengthen the doctor-patient relationship.”
“Patients often have doctors who are passive in their approach, failing to present innovations in the industry that really can help optimize a patient’s visual experience,” Dr. Bazan emphasizes. “They have bad habits of waiting for a patient to bring it up. Patients with proactive doctors are rewarded with options that often enhance vision and improve their lifestyle. Vuity is a great example of this.”
The availability of this treatment has the potential to attract not only new patients but also those who may not be visiting their eye doctor as often as they should. “We have the opportunity to provide eye care for more patients, many of whom have not received it before, such as emmetropic presbyopes,” notes Dr. Barnett. “More than one-third of presbyopic emmetropes have either never seen an eye doctor or have seen one as rarely as every four years. Additionally, 31 million Americans use over-the-counter reading glasses. The approval of a pharmaceutical option for presbyopia is a huge opportunity for eye care and eye care providers to provide eye examinations to diagnose ocular and systemic disease.”
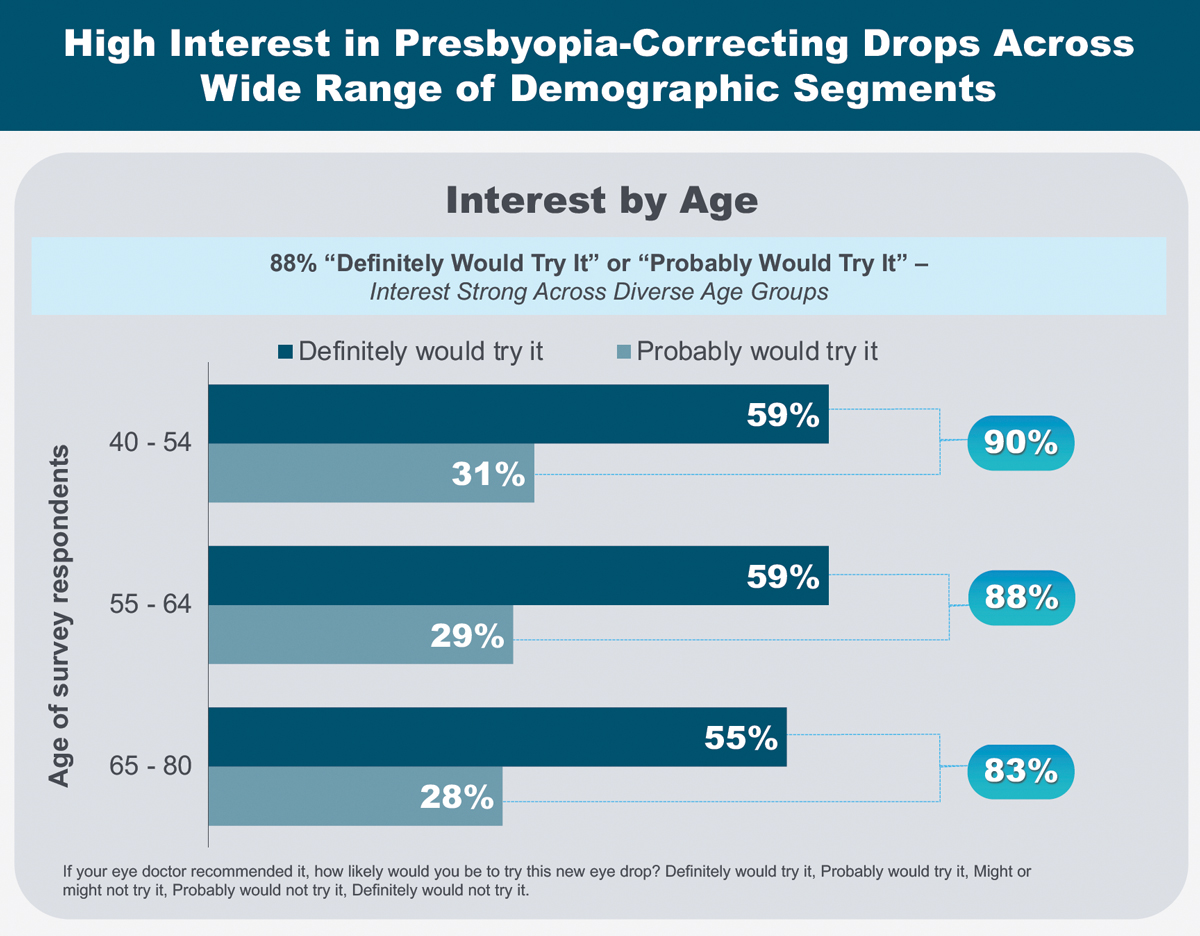 |
|
As part of her AAO 2021 presentation, Dr. Barnett shared patient motivations and expectations surrounding presbyopia-correcting drops. Click image to enlarge. |
The Next Wave of Therapeutics
With other pharmaceutical agents on the horizon for presbyopia, the introduction of Vuity offers a first look at the potential impact of this approach. Time—and further research—will reveal what is possible and if these innovations will change the way optometrists manage presbyopic patients.
Some manufacturers are using cholinergic muscarinic agonists such as pilocarpine in combination with novel ingredients. According to Dr. Shovlin, these new compounds may be effective in enhancing accommodation and depth-of-focus.
Eyenovia has a pilocarpine eye drop in development, with one Phase III study completed and another underway. Additionally, Orasis Pharma has initiated a Phase III clinical trial of its drug CSF-1, which combines pilocarpine and a lubricant.5
“I am aware of at least six other compounds that produce miosis. Most are in the Phase III FDA approval process,” notes Dr. Shovlin. “Lenz Therapeutics is studying the use of aceclidine and Visus Therapeutics is using combined carbachol/brimonidine for a possible dual/synergistic action.”
For a non-miotic approach, Novartis has a lipoic acid/choline ester compound in devemopment, adds Dr. Shovlin. “This drug has been designed to increase accommodation ability,” he explains. “The prodrug reduces dihydrolipoic acid within the lens fibers, causing hydrolysis of the disulfide protein bonds and restoring lens elasticity.”
As this field continues to grow, optometrists are perfectly positioned to educate patients and usher them into a new era of presbyopic treatment. “With the ongoing evolution and advancement of medicine, it is important to be a leader for your patients. It is our responsibility to inform them of every option and help them understand what is available,” notes Dr. Asnaashari. “As with any new innovation, there is a learning curve, but the potential impact is significant. It is truly an exciting time to be an optometrist, and I look forward to what comes next.”
1. First presbyopia eye drop approved. Review of Optometry. October 29, 2021. www.reviewofoptometry.com/article/first-presbyopia-eye-drop-approved. Accessed February 27, 2022. 2. Efficacy study of pilocarpine HCl ophthalmic solution (AGN-190584) in participants with presbyopia (GEMINI 1). ClinicalTrials.gov. clinicaltrials.gov/ct2/show/results/NCT03804268. Accessed February 27, 2022. 3. A phase 3 efficacy study of pilocarpine HCl ophthalmic solution (AGN-190584) in participants with presbyopia (GEMINI 2). ClinicalTrials.gov. clinicaltrials.gov/ct2/show/NCT03857542. Accessed February 27, 2022. 4. U.S. Food and Drug Administration approves Vuity (pilocarpine HCI ophthalmic solution) 1.25%, the first and only eye drop to treat presbyopia (age-related blurry near vision). Abbvie. October 29, 2021. news.abbvie.com/news/press-releases/us-food-and-drug-administration-approves-vuity-pilocarpine-hci-ophthalmic-solution-125-first-and-only-eye-drop-to-treat-presbyopia-age-related-blurry-near-vision.htm. Accessed February 27, 2022. 5. An evaluation of the efficacy and safety of CSF-1 in the temporary correction of presbyopia (NEAR-1). ClinicalTrials.gov. clinicaltrials.gov/ct2/show/NCT04599933?term=orasis&draw=2&rank=3. Accessed February 27, 2022. |