The FDA listed autoimmune disease as a contraindication for LASIK due to concerns about healing. Even so, the procedure may still be a reasonable option in patients whose autoimmune disease is well controlled or inactive, say researchers from the Maloney Vision Institute, Los Angeles, in the August Journal of Cataract and Refractive Surgery.
Admittedly, the association between autoimmune disease, such as rheumatoid arthritis, and corneal disease, such as corneal melt, may justify excluding certain patients from LASIK, the researchers say.
Also, excimer laser manufacturers used autoimmune disease as an exclusion criterion to demonstrate the best safety and efficacy of their lasers during clinical trials. Exclusion criteria during clinical trials then becomes adapted for FDA labeling, the researchers explain.
But, is autoimmune disease really clinically contraindicated in patients who have autoimmune disease?
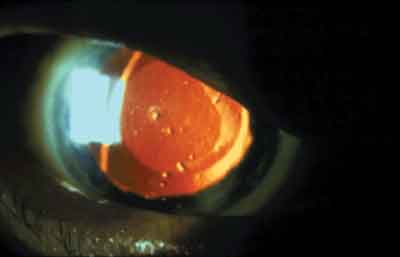 |
| Is LASIK really contraindicated in this patient who has Sjgrens syndrome? |
I think the key to this study is that patients had inactive or stable autoimmune disease, says Paul Karpecki, O.D., of Overland Park, Kan. I think that if [their diseases are] well controlled, they do not respond abnormally and are considered good candidates.
However, Lloyd Pate, O.D., of the University of Houston College of Optometry, says the study design has some shortcomings. Chart reviews always leave some questions about if all the pertinent information was recorded, he says.
A double blind study would be better, he adds.
The researchers themselves say that they cannot rule out a low risk for autoimmune corneal or scleral complications when performing LASIK in these patients. Other limitations, they say, are a lack of data on the incidence or severity of dry eye or dry-eye complaints (a common finding after refractive surgery) and length of follow-up (mean follow-up of 19 months).
|
Are Eye Drops Still Allowed on a Plane? |
|
In response to last month"s terrorist threat, the Transportation Secuirty Administration (TSA) banned all liquids, gels, and arosols from carry-on bags on airplanes. However, travelers can pack these items in checked baggage.
Multiple bottles and non-preserved unit-dose vials are allowed in carry-on luggage as long as the total volume does not exceed 4oz. |

