Scientists have managed to nurture small clumps of the human brain, giving them the ability to grow their own eyes, or at least two functionally integrated optic vesicles that respond to light. These tiny brains, called brain organoids, are self-assembled aggregates resembling the embryonic human brain in both cytoarchitecture and cell type.1 They’re grown from induced pluripotent stem cells (iPSCs), which can be differentiated into a variety of cell types in vitro.
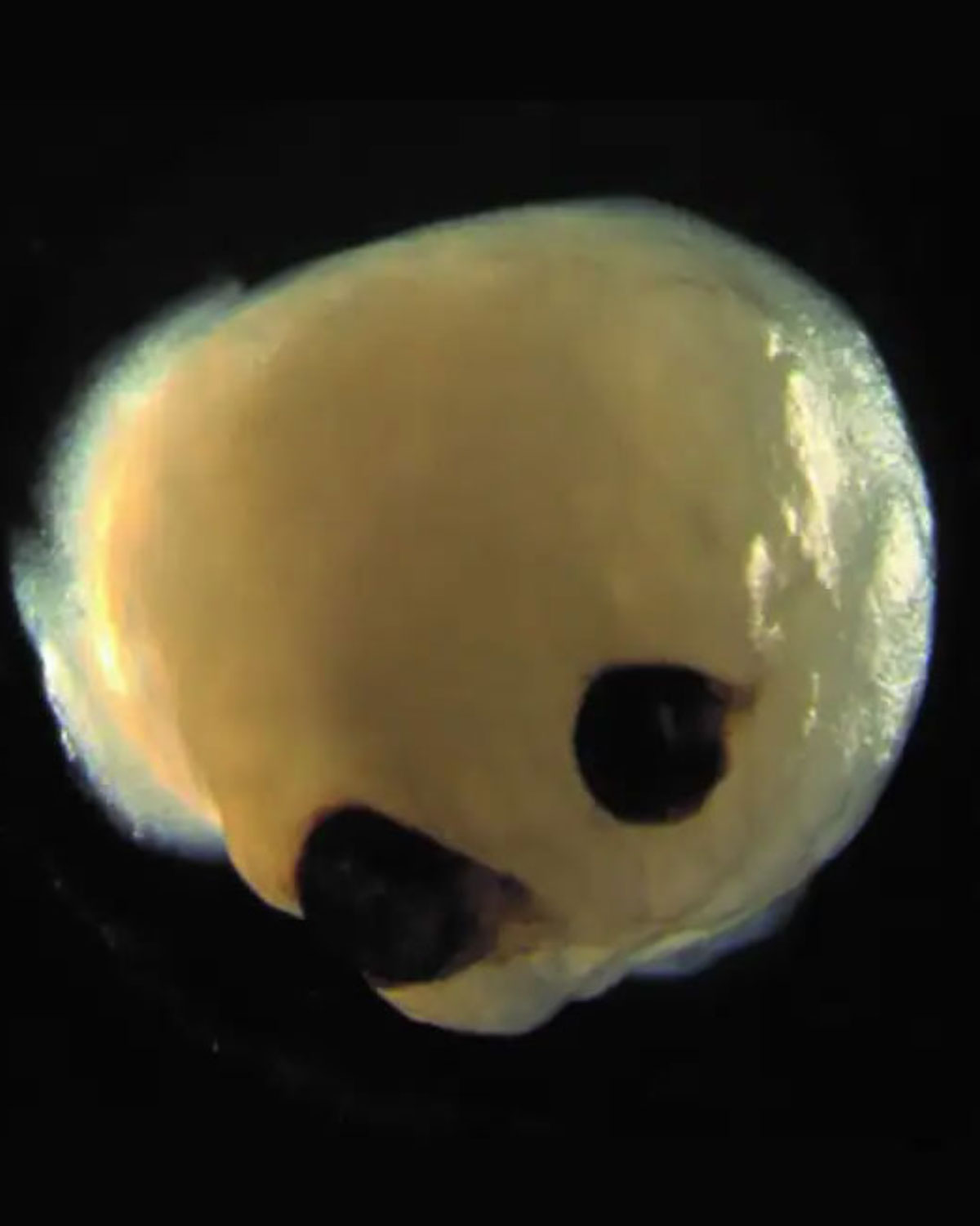 |
| The ambitious project may one day allow scientists to grow artificial retinas suitable for transplantation in blind and visually impaired patients. Image courtesy of Dr. Elke Gabriel, PhD. Click image to enlarge. |
Until now, brain organoids were thought to be “chaotic three-dimensional tissues” unable to follow the self-patterning rules of embryos. However, these brains spontaneously developed bilaterally symmetric optic vesicles in what would be the forebrain region.2 While the eye structures are rudimentary, they have both a lens and a retina and can send signals to the brain tissue.
To encourage the brain organoids to grow eyes, Jay Gopalakrishnan, PhD, of the University Hospital Düsseldorf in Germany, and his team modified an iPSC protocol for differentiating into neural tissue by adding retinoic acid, a vitamin A derivative key for embryonic eye development, 20 days into the brain organoids’ development.
The researchers reported that 72% of 314 brain organoids treated with 60nM of retinol acetate reproducibly attempted to assemble optic vesicles by around day 30, and visible eye structures developed within 60 days. The organoids’ development timeframe parallels that of human embryonic retinal development.
Analysis revealed that the optic vesicles contained primitive corneal epithelial and lens-like cells, retinal pigment epithelial cells, retinal progenitor cells, axon-like projections and electrically active neuronal networks, showing retinal connectivity to the brain. “In the mammalian brain, nerve fibers of retinal ganglion cells reach out to connect with their brain targets, an aspect that has never before been shown in an in vitro system,” said Dr. Gopalakrishnan.
“Interestingly, various light intensities could trigger photosensitive activity of optic vesicle-containing brain organoids, and light sensitivities could be reset after transient photobleaching,” he said. “Thus, brain organoids have the intrinsic ability to self-organize forebrain-associated primitive sensory structures in a topographically restricted manner and can allow interorgan interaction studies within a single organoid.”
The researchers say the relatively short time to generate eye-like structures is crucial for the future of in vivo developmental biology because such a timeframe will allow for multiple experimental setups. Brain organoids containing optic vesicles will pave the way for “personalized organoids and RPE sheets for transplantation,” they said. They believe such organoids will help to model retinopathies emerging from early neurodevelopmental disorders.
1. Qian X, Song H, Ming G. Brain organoids: advances, applications and challenges. Development. 2019;146:8. 2. Gabriel E, Albanna W, Pasquini G, et al. Human brain organoids assemble functionally integrated bilateral optic vesicles. Cell Stem Cells. July 10, 2021. [Epub ahead of print]. |

