 |  |
Gone are the days when there were no surgical options for patients with mild-to-moderate glaucoma. With the advent of minimally invasive glaucoma surgeries (MIGS), there now exists an ever-expanding list of options to treat these patients surgically and reduce dependence on drops.
FDA-approved in August 2018, the Hydrus Microstent (Ivantis) is a long, flexible structure roughly the size of an eyelash that research suggests can effectively lower intraocular pressure (IOP). It is only indicated for patients with mild-to-moderate open-angle glaucoma who are undergoing cataract surgery. It is not indicated for those with other forms of glaucoma or birth irregularities of the anterior chamber.
Process and Progress
Taking an ab interno approach, the surgeon carefully places the 8mm device using incisions created during cataract surgery and a preloaded injector. It is inserted through the trabecular meshwork and into Schlemm’s canal so that it spans 90° of the canal. There is no need for targeted insertion, as the length of the device accounts for one-fourth of Schlemm’s canal, ensuring that multiple collector channels are targeted.
By bypassing the trabecular meshwork, the microstent redirects the flow of aqueous fluid and allows it to drain directly into Schlemm’s canal for easier outflow. The flexible design of the device allows for gentle expansion of the canal, leaving the collector channels unaffected.
This newer MIGS procedure has achieved successful clinical trial results. The Horizon study found 78% of Hydrus Microstent patients were able to remain medication-free after two years, a 30% improvement compared with cataract surgery alone.1 This study further showed that 85.9% of Hydrus Microstent patients experienced an IOP reduction of 20% or more at one year and 77.2% retained this outcome after two years, indicating that the implant can both lower and maintain improved IOP levels.1
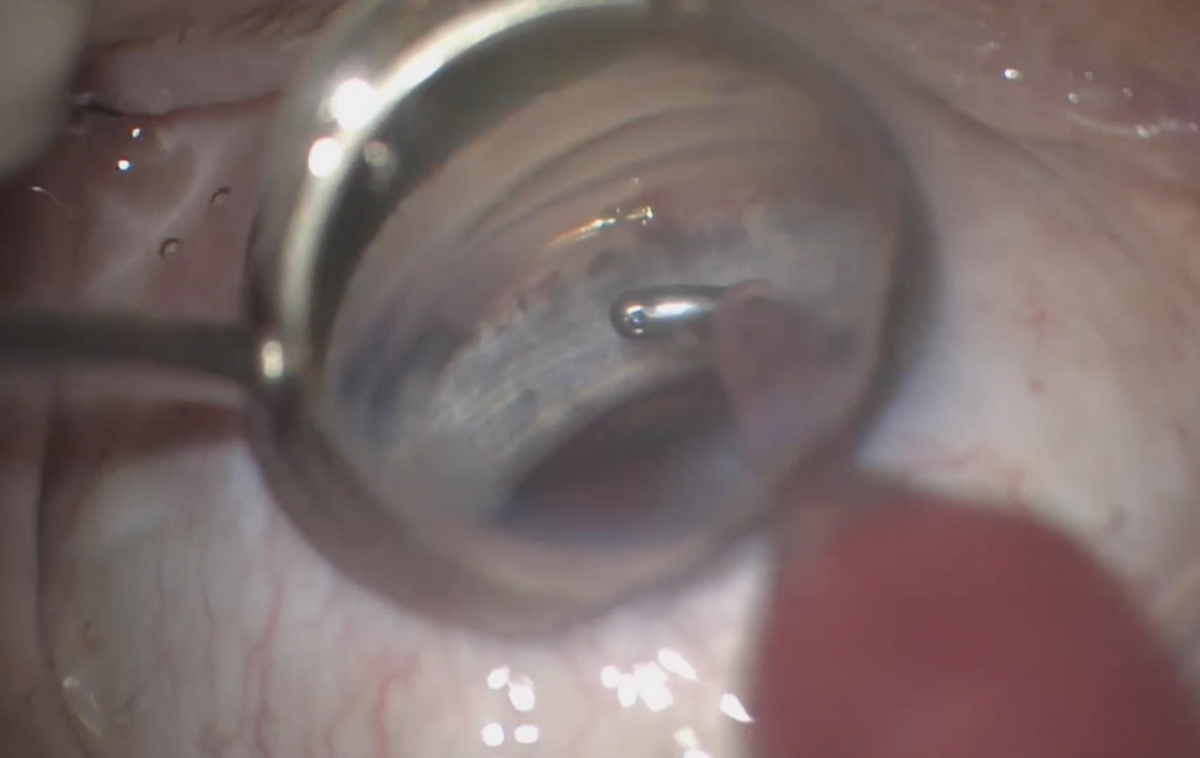 |
| After good stent placement is confirmed, the MIGS procedure is complete. Click image to enlarge. |
Pros and Cons
One advantage of Hydrus is its safety profile, as procedures that bypass the trabecular meshwork and act on the canal are among the safest. However, all MIGS come with risks. The most common adverse events associated with this device include non-persistent anterior uveitis requiring a change in post-op steroid treatment, microstent malposition and microstent obstruction with or without peripheral anterior synechiae. All tend to occur at low incidences.2
Other complications include hyphema or microhyphema, transient anterior chamber shallowing, iris erosion, pupil peaking and early hypotony (IOP less than 6mm Hg within two weeks after surgery) accompanied by corneal folds.
Since the device is designed to enhance normal outflow mechanisms, post-op patients are protected against episodes of hypotony that sometimes occur after glaucoma surgery.
The device’s effect on IOP is apparent early after surgery, allowing the surgeon to make a decision regarding the discontinuation of drops shortly after the procedure.
Research shows this low-risk MIGS can provide IOP-lowering effects as far out as four years after insertion. So far, it has proven to be yet another useful weapon in the battle against glaucoma.
1. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON Study. Ophthalmology. 2019;126(1):29-37. 2. Summary of safety and effectiveness data (SSED): Hydrus Microstent. US Food and Drug Administration. www.accessdata.fda.gov/cdrh_docs/pdf17/P170034B.pdf. August 10, 2018. Accessed May 8, 2020. |

