 |
History
A 65-year-old woman presented to the ophthalmology department as an emergency consultation with a chief complaint of recently noticed vision changes “next to her nose” with new floating spots. She was a poor historian, not recalling any ocular or systemic illnesses, and confessed she had not been to a medical or eye doctor “in years.” She reported no ocular or systemic histories, took no medications and denied allergies of any kind.
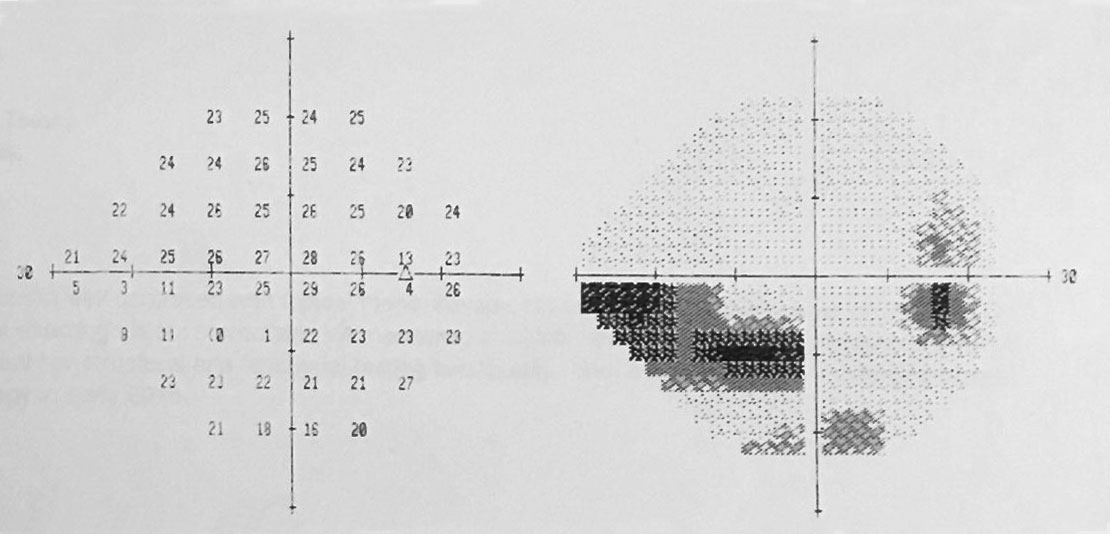 |
| Automated perimetry yielded these field results. Click image to enlarge. |
Diagnostic Data
Her best entering acuities were 20/30 uncorrected at distance and 20/30 at near with use of a reading spectacle. There was an afferent defect OD; however, color vision was intact with no red desaturation and no brightness comparison loss.
The facial Amsler grid uncovered general constriction without any pathognomonic neurological symmetry, OD>OS. Extraocular motilities were intact. Refraction was negligible and her near acuity improved when her reading addition was increased to +2.50 DS.
Biomicroscopic exam of the anterior segment found normal structures with no evidence of iritis, rubeosis, exfoliation, pigment dispersion or gross trauma. Intraocular pressures measured 18 OU with Goldmann applanation.
The patient had gonioscopy completed OU to rule out evidence of previous trauma (angle recession), pseudo or true exfoliation syndrome, pigment dispersion, synechial closure or evidence of plateau iris and both nerves were photodocumented.
OCT was completed to gather information regarding optic nerve fiber layer analysis and macular vulnerability zone thickness. Additional history questions included presence of sleep apnea, unusual routine such as yoga, contact sports or gymnastics and whether there was ever an episode of blunt trauma or blood loss.
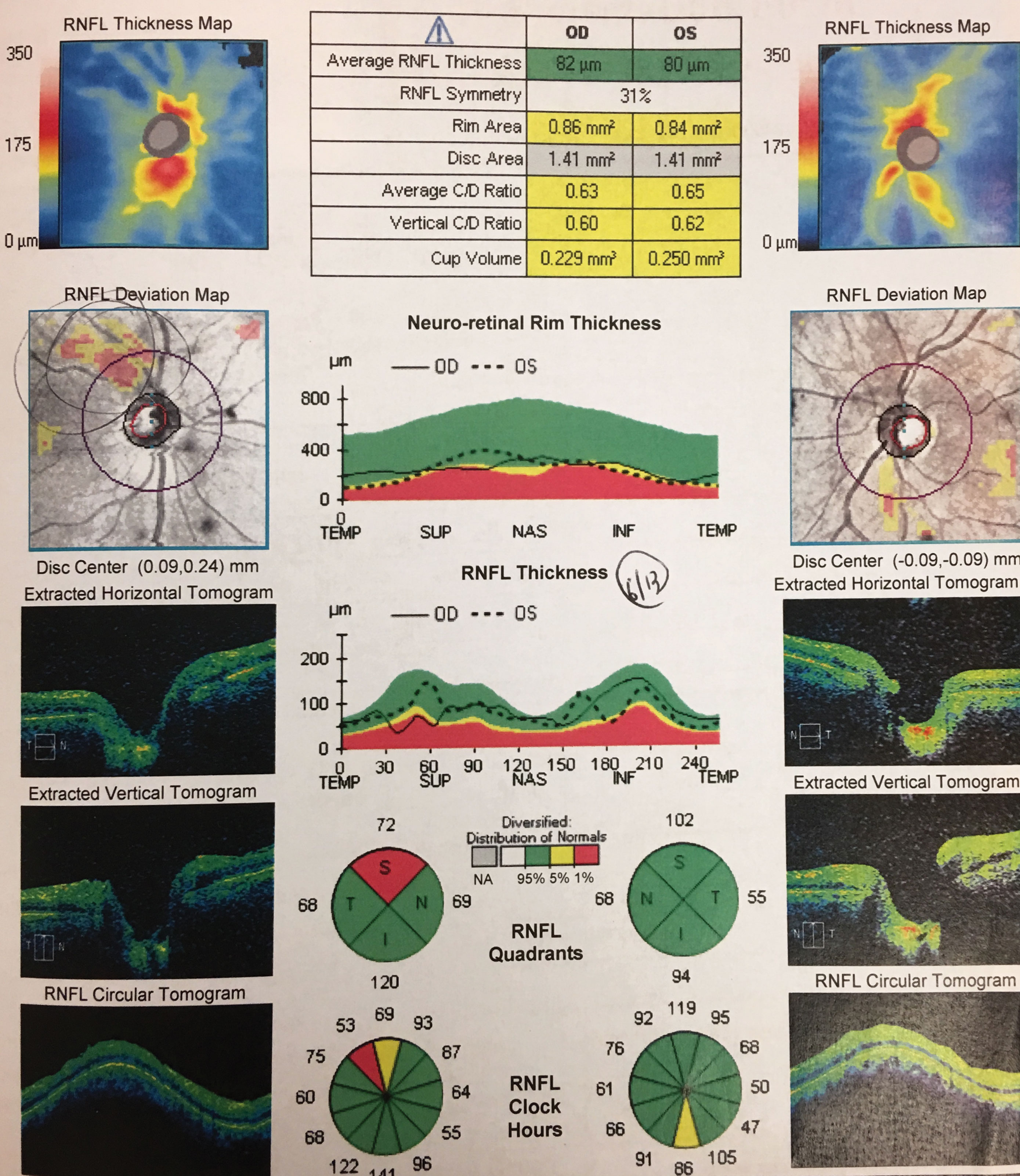 |
| RNFL scans OS and OD. Click image to enlarge. |
Discussion
The diagnosis this month is normal tension glaucoma (NTG) by exclusion. NTG is a multifactorial disease where mechanical stresses and vascular alterations of blood flow to the optic nerve head create retinal ganglion stress and cell loss.1-3 Several IOP-independent factors such as glutamate toxicity, oxidative stress, autoimmunity, genetics, cardiac issues, blood loss and vascular dysregulation have been considered in the pathogenesis of NTG.1-3
Normal tension glaucoma is a diagnosis of exclusion, as non-glaucomatous sources exist that can produce cupping. The phenomenon of optic disc cupping has two pathophysiologic components: prelaminar thinning and laminar deformation. Prelaminar thinning is defined as the portion of cup enlargement that results from thinning of the prelaminar tissues due to physical compression and/or loss of retinal ganglion cell axons from pathologies which are not IOP related. Laminar deformation or laminar cupping results from permanent intraocular pressure (IOP)–induced deformation of the lamina cribrosa and peripapillary scleral connective tissues after damage and/or remodeling.4
One defining characteristic of glaucomatous cupping is that is has the reputation of leaving an otherwise well-fed rim of ganglion cell tissue intact (pink) as the deeper neural and connective tissues deform, remodel or mechanically fail. Glaucomatous cupping often can be identified by its distinctive pattern (verticalization with focal notching, indicating concentrated loss of ganglion cells in one specific area) which is produced by a combination of the physical anatomical traits of the lamina cribrosa as persuaded by the pathophysiologic processes that are induced by chronically increased IOP or by poor optic nerve perfusion inducing related connective tissue stress and strain.4-7
Since arteritic and non-arteritic ischemic optic neuropathy and compressive optic neuropathies can cause “pale” cupping, NTG is a diagnosis of exclusion. Also, hypovolemic optic neuropathy secondary to acute blood loss can produce cupping without the chronic process of glaucoma. Cases of normal tension glaucoma should be examined with scrutiny. Work up for non-glaucomatous sources should be completed in any case that is not clear-cut.
Intraocular pressure reduction is the only modifiable risk factor that can be used to restrict progression. Recently, new treatment strategies have been proposed to improve the control of the disease. Neuroprotection is a rapidly expanding area of research, which represents a promising tool.1-3
We placed the patient on topical Travatan Z (Allergan) at bedtime in both eyes to provide some “first-aid” and arranged for them to see the neuro-ophthalmologist for a suggested laboratory work up and neuroimaging to rule out non-glaucomatous sources of optic neuropathy. They will see the glaucoma specialist if the diagnosis of NTG is confirmed to rule out surgical solutions.
| 1. Esporcatte BL, Tavares IM. Normal-tension glaucoma: an update. Arq Bras Oftalmol. 2016;79(4):270-276. 2. Mastropasqua R, Fasanella V, Agnifili L, et al. Advance in the pathogenesis and treatment of normal-tension glaucoma. Prog Brain Res. 2015;221(5):213-232. 3. Song BJ, Caprioli J. New directions in the treatment of normal tension glaucoma. Indian J Ophthalmol. 2014;62(5):529-537. 4. Burgoyne C. The morphological difference between glaucoma and other optic neuropathies. J Neuroophthalmol. 2015;35 Suppl 1(0 1):S8-S21. 5. Burgoyne CF, Downs JC. Premise and prediction-how optic nerve head biomechanics underlies the susceptibility and clinical behavior of the aged optic nerve head. J Glaucoma. 2008;17(4):318-328. 6. Moghimi S, Zangwill LM, Manalastas PIC, et al. Association Between Lamina Cribrosa Defects and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. JAMA Ophthalmol. 2019;137(4):425-433. 7. Abe RY, Gracitelli CP, Diniz-Filho A, Tatham AJ, Medeiros FA. Lamina Cribrosa in Glaucoma: Diagnosis and Monitoring. Curr Ophthalmol Rep. 2015;3(2):74-84. |

