The interpalpebral space of the conjunctiva frequently develops irregular tissue changes that are, mostly, minimally pathologic or not pathologic at all. This location is exposed to ultraviolet (UV) radiation and atmospheric irritants and is susceptible to dryness; thus, lesions such as pingueculae and pterygia are commonly encountered ocular surface irregularities and generally draw minimal attention on exam.
Squamous neoplastic disease, however, is occasionally encountered. Invasive conjunctival squamous cell carcinoma is often histologically preceded by conjunctival intraepithelial neoplasm (CIN), which represents the most frequently encountered conjunctival neoplastic growth. These lesions—often misdiagnosed as more typical ocular surface growths—are slowly progressive, locally invasive and have no metastatic potential. However, they may occasionally cause significant local ocular surface damage and can progress to the more invasive squamous cell carcinoma. Both the diagnosis and treatment of CIN can be challenging. This article offers insights for ODs who may not encounter CIN too often and would like a clinical overview.
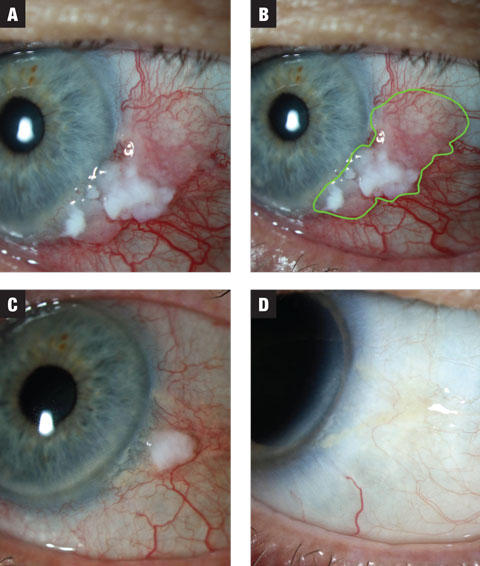 |
| Fig. 1a. In case 1, the lesion has both leukoplakic and gelatinous zones. Treatment was initiated at MMC 0.02% QID. Fig. 1b. Estimated extent of lesion highlighted. Fig. 1c. After the first three-week course, the lesion shows dramatic reduction in size. The patient was placed on a washout period of two weeks and instructed to anticipate a second course of MMC. Fig. 1d. Lesion showed complete resolution following washout. Click image to enlarge. |
CIN Development
The genesis of an epithelial cancerous cell population, or carcinoma, requires a series of changes to the cells’ behavior. Cells that exhibit this dysplasia show disorganized growth and maturation, resulting in an overabundance of immature cells which is contrasted by a concomitant relative paucity of mature cells of this line. While dysplasia is a reversible process, dysplastic cells may then undergo further mutation, resulting in neoplastic transformation, whereby the cells become insensitive to growth inhibitors and become invasive.
Neoplastic carcinomas may be locally confined by basement membrane—known as carcinoma in situ—or may be invasive, characterized by the lesion breaking through the respective basement membrane and spreading locally. This staging, however, does not refer to two separate entities; rather, carcinoma in situ represents a preliminary step along the same continuum of neoplasia. If left alone, carcinoma in situ may eventually become invasive.
While it is possible for squamous carcinoma to skip the step of CIN, it is frequently the midpoint in the disease’s etiology. CIN is an abnormal line of conjunctival (and possibly corneal) epithelial cells and represents either simple dysplasia (partial thickness of epithelial tissue) or carcinoma in situ when the lesion is full thickness.1-4 CIN is part of the spectrum of neoplastic disorders of the conjunctiva and cornea known collectively as ocular surface squamous neoplasia (OSSN). If CIN becomes invasive by breaking through basement membrane, it is reclassified as invasive squamous cell carcinoma (SCC).
Though uncommon, CIN is the most frequently encountered conjunctival neoplasm in the United States.4 Risk factors for the development of CIN, and all forms of OSSN, are UV exposure (particularly UV-B), male gender, exposure to petroleum products, heavy tobacco smoke, human immune deficiency virus (HIV) and human papillomavirus (HPV) type 16, though this last risk remains controversial.1,3-5 CIN prognosis is usually good, except in cases when the growth is unusually large. Once the lesion breaks basement membrane and becomes invasive, prognosis is worse and often calls for aggressive treatment such as enucleation or exteneration. Even in these cases, risk of distant metastasis is low.
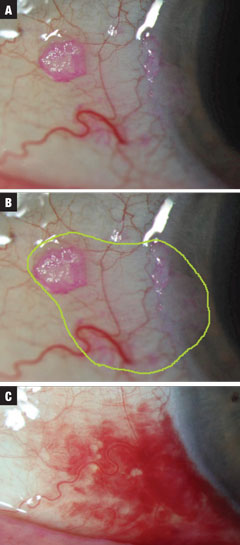 |
| Figs. 2a-c (from top to bottom). Case 2 involved a small suspected CIN lesion discovered incidentally on exam. Treatment was initiated at INF-a2b QID until the 10mL bottle was empty. The estimated total extent of neoplastic margins is highlighted in green. Finally, resolution was seen, with concurrent SCH related to valsalva (not medication induced). Treatment required one month at one million IU QID and then 10 days QID at three million IU of INF-a2b. Click image to enlarge. |
Clinical Presentation
The clinical appearance of CIN is that of an abnormal, slightly elevated, fleshy mass, typically located at the interpalpebral limbal zone (95% of all lesions).6 The predilection for this area is likely due to its features. UV-B exposure is greatest in the interpalpebral zone, and the stem cell zone of the limbus is a transitional space between the corneal and conjunctival epithelium. This transition zone likely confers special risk to the tissue for undergoing dysplasia.
The conjunctival lesions may be gelatinous, papillary or, less commonly, leukoplakic, which occurs as a result of hyperkeratosis. These lesions are usually well defined and will show some degree of feeder vasculature.3,7,8 Ninety-five percent of cases will involve the limbus.6 Corneal manifestations show similar whitening and can exhibit gray, fimbriated (i.e., finger-like) epithelial projections from the limbus. Classically, the lesions will demonstrate positive staining or stippling with rose bengal, which can be useful in the clinical differentiation of CIN from other conjunctival lesions.8,9
Despite relatively prominent findings on paper, CIN and SCC lesions can be challenging to differentiate from more normal growths of the conjunctiva, such as pingueculae, pterygia and nevi. This can lead to misdiagnosis in up to 60% of cases, even when evaluated by experienced clinicians—making biopsy with histologic assessment potentially valuable.3
Diagnostic Testing
Historically, testing included excisional biopsy performed at the time of surgical removal. However, physicians are now using chemotherapy as a primary treatment modality, which may limit access for biopsy.
A newer diagnostic tool is impression cytology, which is performed by applying a filter paper to the lesion, removing the superficial epithelium and allowing minimally invasive histological studies. This is roughly 80% sensitive, though false negatives remain its weakness, because the collecting paper may be stymied by excess keratosis of the lesion; repeat attempts are generally more successful.8 One study shows ultra-high resolution OCT, though not currently available, is very sensitive in the differentiation of OSSN and pterygium on the basis of the lesion’s epithelial thickness.10
Treatment
The traditional treatment of CIN is excision with margins of 1mm to 5mm, depending on the extent and history of primary and recurrent lesions. Corneal involvement is debrided with a surgical blade, and cryotherapy is applied to the bed and conjunctival edges. The sclera is left bare. This is usually successful, but recurrences are reported between 10% and 52%, depending on the rate of clear surgical margins as determined by postoperative histology.6-11 The rate drops to 5% if clear margins are achieved; unfortunately, studies show it is difficult to guarantee clear margins.8,9 These numbers would seem to support the use of the widest margins possible; however, there are consequences of extensive conjunctival excision: cicatricial changes, limbal stem cell deficiency, scleral melt, significant disturbance to the tear film and irregular changes in corneal astigmatism.
Topical Options
More recently, topical chemotherapeutics have become popular to manage CIN, as they have the benefit of treating the entire ocular surface and, when used judiciously, seem to be well tolerated.
Mitomycin. Discovered in the 1950s, mitomycins are fermentation byproducts of Streptomyces caespitosus. Mitomycin-C (MMC), the last of these molecules discovered, is an antimetabolite used outside of eye care as an anti-tumor chemotherapy agent.12,13 MMC works as an alkylating agent that prevents DNA splitting during cellular mitosis, an alteration that is extremely toxic.1 Even one of these crosslinks can be fatal to a cell.13 Additionally, MMC may generate reactive oxygen species and increase the synthesis of tumor necrosis factor (TNF).
Though it has its roots in oncology, MMC use has become more widespread, particularly within eye care, in attempts to limit excessive postoperative scar formation. Optometrists who comanage surgery patients may be familiar with its intraoperative use to prevent scarring in patients who have undergone a glaucoma filtering procedure and its use in PRK to prevent corneal haze. Since its first OSSN-associated use in the mid 1990s, MMC has proven efficacious, with success rates between 82% and 100%. Dosing is typically 0.04% concentration given QID in one-week-on, one-week-off cycles, or a 0.02% concentration is continuously dosed in four-week treatments.4 Medication toxicity is greater with prolonged treatments or higher concentrations
As MMC is an especially potent, potentially dangerous medication, its side effects—limbal stem cell deficiency and scleral melt, for example—may be severe. More frequently encountered side such as corneal and conjunctival epitheliopathy and conjunctival injection are transient.4,14 When used, MMC requires careful follow up and cessation of medication when any significant side effect is suspected.
5-Fluorouracil (5-FU). This is another antimetabolite used in the treatment of dermatologic tumors, and also by glaucoma surgeons. Its mechanism blocks DNA synthesis by interfering with the enzyme thymidylate synthase. In the treatment of OSSN, 5-FU is generally dosed at a concentration of 1% QID one-month-on, one-month-off until resolution. Most cases resolve with one or two cycles, though some take as long as five. Epithelial toxicity, resulting in erosion or sloughing, is common. In one study, the treatment duration was set as the time until sloughing occurred, with time off treatment lasting until re-epithelialization. Despite this painful complication, side effects of 5-FU do not seem to be as severe as MMC, and recurrence rates are generally reported as equivalent.4,14
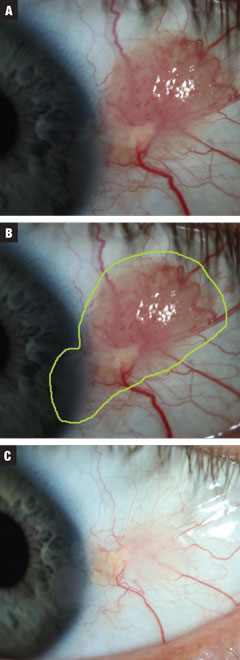 |
| Fig. 3a. Case 3 involved a moderate-sized presumed papillomatous CIN. The patient was started on treatment INF-a2b three million IU for 10 days. Fig. 3b. Estimated total lesion size. Fig. 3c. Post treatment. Near complete resolution of growth with continued irregular corneal epithelium. Against advisement, the patient opted to observe at this time. Click image to enlarge. |
Interferon alpha 2b (INF-a2b). Interferons are a subgroup of naturally occurring inflammatory proteins known as cytokines. They are produced by activated immune cells and have variable antineoplastic, antiviral and antimicrobial effects. The first reported use of INF-a2b in 1994 yielded successful resolution of a CIN lesion with its topical use.5 Since then, INF-a2b has been used both topically and subconjunctivally, with good effect, in the treatment of OSSN.
Perilesional dosing has the greatest efficacy, with 87% to 100% resolution over a five-week period (with injections given up to three times per week).4,14,15 Multiple injections are necessary to obtain this level of efficacy, which results in up to 100% of patients developing systemic myalgias and fever.5,14 Topical dosing is roughly equivalent to MMC in effectiveness.14,15 It is given at a concentration of one million international units (IU) per millimeter and dosed QID for one month beyond clinical resolution. When resolution does not occur, the concentration may be increased to up to three million IU, also QID. Generally, regardless of the treatment concentration, compounding pharmacies will prepare the bottle from a single preparation of one million IU. This one million IU preparation can be concentrated further by reducing the amount of carrier to the bottle. Side effects of topical INF-a2b are mild, with hyperemia and follicular conjunctivitis being frequently reported; keratitis is reported less commonly.4
Topical vs. Surgery
Though biopsy and surgical excision were the historic treatment of choice, topical chemotherapeutics, which have some theoretical benefits over surgery, have gained some acceptance as front line therapy. With surgery, it is impossible to guarantee clear margins—a feature required for the best prognosis. The greater the amount of tissue removed to ensure clear margins, the greater the potential for long-term disruption of the ocular surface. Topical agents treat the entire ocular surface, eliminating the need to identify margins and allowing effective treatment of neoplastic cellular populations that could not be plainly identified with microscopic analysis—a benefit that may lead to the pairing of topical agents with surgical excision as a total treatment protocol for large lesions. The drawback to topical therapy, of course, is that since the entire ocular surface is exposed to the medication—technically an overtreatment—complications may arise and, in the case of MMC in particular, those complications though rare, may be severe.
As far as efficacy is concerned, all topical chemotherapy agents for the management of OSSN perform reasonably well. INF-a2b has undergone head-to-head efficacy studies against surgical resection and has been shown to eliminate the lesion at comparable rates, and recurrence rates may actually be lower for INF-a2b relative to resection.4 Follow-up studies report that its use is more appropriate in simple or small lesions, while surgery is preferable in cases of more advanced lesions, though topical therapy may be preferable for recurrent lesions.4,15
MMC generally has the shortest treatment course, but is the most toxic and carries the greatest risk of complications. 5-FU has a middling side effect profile and treatment duration; it is also the least expensive of the group. INF-a2b has superior tolerability, but is often associated with longer treatment and greater cost.4,13-16
Cost certainly may be a consideration when deciding to use topical chemotherapeutics. Insurance companies will sometimes refuse to cover these medications, reporting their use as experimental. In short courses of treatment and comparing only total healthcare expenditure, topical chemotherapeutics are less expensive than surgery; however, they can exceed the expense of surgery after several courses.
This possible stumbling block aside, given the efficacy, ability to avoid surgery and general tolerability, when used in a monitored fashion topical chemotherapeutics appear to be a good option for the treatment of conjunctival intraepithelial neoplasm lesions.
Dr. Bronner is a staff optometrist with Pacific Cataract and Laser Institute in Kennewick, Wash.
|
1. Kiire CA, Dhillon B. The aetiology and associations of conjunctival intraepithelial neoplasia. Br J Ophthalmol. 2006:90(1);109-13. 2. Birkholz ES et al. Treatment of ocular surface squamous cell intraepithelial neoplasia with and without mitomycin C. Cornea. 2011;30:37-41. 3. Lee GA, Hirst LW. Ocular surface squamous neoplasia. Survey of Ophthalmology. 1995; 39:429-50. 4. Nelson KD, McSoley JJ. Clinical findings and management of conjunctival intraepithelial neoplasia. Optometry. 2011; 82:15-21. 5. Nanji AA, Sayyad FE, Karp CL. Topical chemotherapy for ocular surface squamous neoplasia. Current Opinion in Ophthalmology. 2013; 24:336-42. 6. Warner M, Jakobiec F. Squamous Neoplasms of the Conjunctiva. In: Krachmer JH, Mannis MJ, Holland EJ eds. Cornea. 2nd ed. St Louis: Mosby; 2004:557-70. 7. Rapuono CJ, Luchs JI, Kim T. Conjunctival and External Disease. Anterior Segment: the requisites. Ed. Krachmer, JH. Mosby. 2000;33-6. 8. Huerva V and Ascaso FJ. Conjunctival Intraepithelial Neoplasia – Clinical Presentation, Diagnosis and Treatment Possibilities. Srivstava S (Ed.) ISBN: 978-953-307-987-5, InTech. 9. Nelson KD, McSoley JJ. Clinical findings and management of conjunctival intraepithelial Neoplasia. Optometry. 2011;82:15-21. 10. Kieval JZ1, Karp CL, Abou Shousha M, et al. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterigia. Invest Ophthalmol and Vis Sci. 2011; 52:1741. 11. Peksayar G, Soyturk MK, Demiryont M: Longterm results of cryotherapy on malignant epithelial tumors of the conjunctiva. Amer J of Ophthalmol. 1989;107: 337. 12. Santhiago MR, Netto MV, Wilson SE. Mitomycin C: Biological effects and use in refractive surgery. Cornea. 2012; 31:311-21. 13. Tomaz M. Mitomycin C: small, fast and deadly (but very selective). Chem and Biol. 1995; 2:575-9. 14. Majmudar PA, Epstein RJ. Antimetabolites in ocular surface neoplasia. Curr Opin in Ophthalmol. 1998; 9:35-9. 15. Besley J, Pappalardo J, Lee GA. Risk factors of ocular surface squamous neoplasia recurrence after treatment with topical mitomycin C and interferon alpha 2b. Amer J of Ophthalmol 2014; 157:287-93. 16. Boehm M and Huang AJW. Treatment of recurrent corneal and conjunctival intraepithelial neoplasia with topical interferon alpha-2b. Ophthalmol. 2004; 111:1755-61. |

