Innovations in Eye CareCheck out the other feature articles in this month's issue:A Light in the DARC: Seeing Glaucoma Before it Strikes Bioengineering the Retinal Pigment Epithelium Can We Lower IOP with Glasses? While You Were Sleeping |
For years, researchers looked for ways to maximize the effects of topical treatments for chronic ocular conditions such as dry eye and uveitis. While eye drops are simple in theory, in practice they come with a host of issues that hamper their efficacy. Eye drops deliver a volume of 30µl to 50µl of medication to the eye, most of which drains into the nasolacrimal system or spills onto the lower lid—meaning only 1% to 5% of the drug is actually absorbed by the eye upon instillation.1 This necessitates more frequent dosing, which often results in poor compliance, reduced treatment effect, local tolerability issues and negative outcomes.
A novel drug delivery system that uses a small electrical current—known as iontophoresis—may soon reduce these limitations by allowing eye care practitioners to achieve topical delivery of concentrated medication through a simple, in-office process.
As the current (Dr. Wirostko) and former (Dr. Raizman) chief medical officers of EyeGate Pharmaceuticals—the company developing this technology—we have had the privilege of helping bring this exciting concept closer to realization as a commercially viable product. Here, we share what we have learned and where we anticipate the technology going.
A Shocking Discovery
While the concept of iontophoresis dates back to the early 1900s, only within the last few years have controlled clinical studies tested its efficacy.2 Iontophoresis is a noninvasive process that allows a greater bioavailability of a drug to reach the anterior and posterior segments than is normally possible with topical application.3 It is also safer than systemic dosing, which exposes patients to a higher risk of system-wide adverse effects, and intravitreal injection, which is invasive and increases the risk of infection.3
Ocular drug delivery through iontophoresis follows the principle that like charges repel and opposite charges attract.4 This allows more effective penetration through ocular tissues by promoting the movement of a charged drug across biological membranes and enabling the delivery of negatively or positively charged therapeutics through tissues to targeted areas.3,4
The amount of the drug that enters the eye can be controlled in two ways: the strength of the current and the duration of the treatment. By controlling the drug amount, the clinician can deliver therapeutic levels of the drug into the eye while minimizing systemic absorption.3 In a Phase III trial in anterior uveitis patients, iontophoresis drug delivery reduced application of conventional topical drugs by almost 98%—two iontophoretic treatments were equivalent to 154 eye drops.5
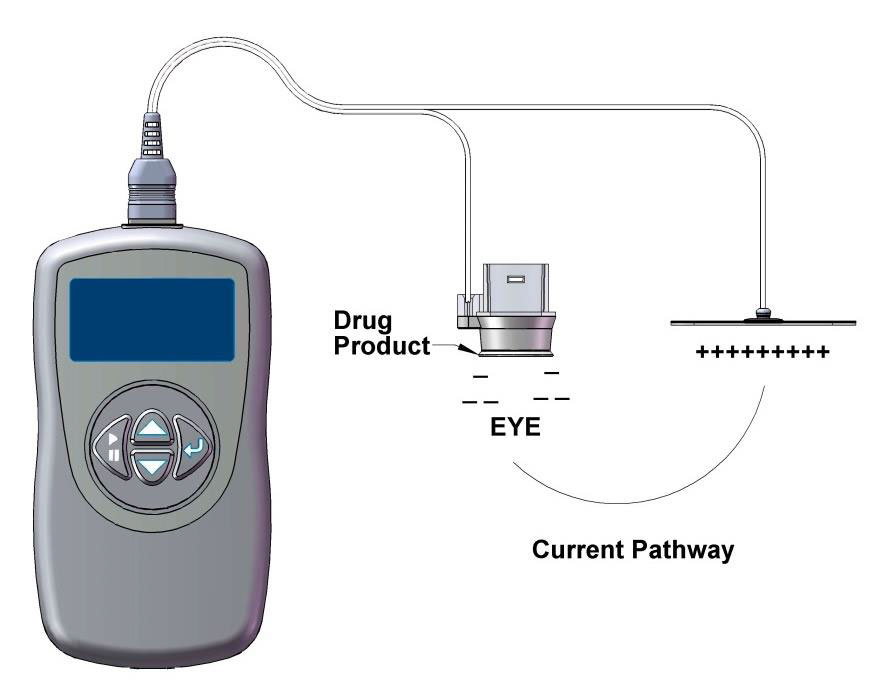 |
| The EyeGate II includes an applicator placed on the conjunctiva and a generator connected to an electrode attached to the patient’s forehead. |
Going Electric
The system developed by EyeGate Pharmaceuticals, called EyeGate II, uses an electrical field generated by a low-level current to enhance the mobility of charged particles.3 The device combines an applicator placed on the conjunctiva at the limbus and a generator connected to an electrode attached to the patient’s forehead.3 The generator creates an electric field inside the applicator and an opposite charge on the electrode. The drug resides in the applicator and is propelled through the conjunctiva and sclera during the periods of electrical stimulation.3
The therapeutic agent, EGP-437, is a 40mg/mL dexamethasone phosphate (DP) formulation specifically developed for use in iontophoresis to treat inflammatory conditions such as anterior uveitis.5 This drug and the EyeGate II drug delivery system are under investigation.5
Study Notes
Upon assessing the safety and efficacy of EGP-437 in dry eye treatment, researchers found that ocular iontophoresis of the drug demonstrated statistically and clinically significant improvements in signs and symptoms within a controlled adverse environment model.6
The participants (103 dry eye patients) were randomly divided into three groups—low-dose, high-dose and placebo arms—with the following characteristics: 7.5mA/minute at 2.5mA with DP in the reservoir, 10.5mA/minute at 3.5mA with DP in the reservoir and 10.5mA/minute at 3.5mA with no DP in the reservoir.6 Patients were assessed seven times over a three-week period prior to and following treatment with the EyeGate II system.
Although the primary endpoints concerning improvement in signs and symptoms at the fifth visit were not achieved, both therapeutic groups showed some statistically significant gains at various other time points.6 The low-dose protocol appeared more effective than the high-dose regimen; the researchers proposed that this may be because the high-dose application drove the therapeutic agent too deeply into the globe, reducing efficacy relative to the lower-dose effect.
The low-dose group also exhibited statistically significant improvements in corneal staining, ocular protection index and ocular discomfort during follow-up visits.6 Clinical findings show that treatment-emergent adverse events (AEs) were experienced by 87% of patients and were consistent across all treatment groups.6 Most were mild, and no severe AEs were observed.6
Researchers from EyeGate also evaluated the toxicokinetics and tolerability of DP by transscleral iontophoretic delivery to rabbit eyes in a study that ultimately found repeated treatments might be safe to treat inflammatory ocular disorders that require prolonged or repeated corticosteroid therapy.7 Subjects received EGP-437 transsclerally once biweekly for 24 consecutive weeks at doses of 10mA/minute, 14mA/minute and 20mA/minute.7 The regimen was well-tolerated, and side effects either were expected reactions to the ocular applicator or the iontophoresis process or arose from factors unlikely related to the treatment.7 They note that there was no effect on intraocular pressure (IOP), electroretinography or histopathology attributable to the medication or the iontophoresis treatment.7
One such circumstance requiring repeated corticosteroid use that may benefit from iontophoresis is post-cataract surgery. Visual recovery after cataract surgery can be delayed by inflammation, which can be caused by topical corticosteroids. In an effort to learn whether iontophoresis can help, a study presented at ARVO 2017 by Dr. Wirostko sought to determine the safety and efficacy of EGP-437 in managing post-surgical inflammation and pain.8
The study found that patients receiving the 4.5mA/minute and the 14mA/minute doses exhibited the best results, with 20% to 30% achieving an anterior chamber cell count of zero at day seven and 70% to 80% at day 28, 70% of 4.5mA/minute patients and 90% of 14mA/minute patients reporting no pain on day one and 0% experiencing elevated IOP.8 Post-op pain and inflammation were managed as early as days one and seven, respectively.8 The study concluded that the iontophoretic delivery of EGP-437 is a safe and effective way to deliver adequate amounts of steroids and has the potential to eliminate the daily need for corticosteroid eye drops in post-cataract surgery patients.8
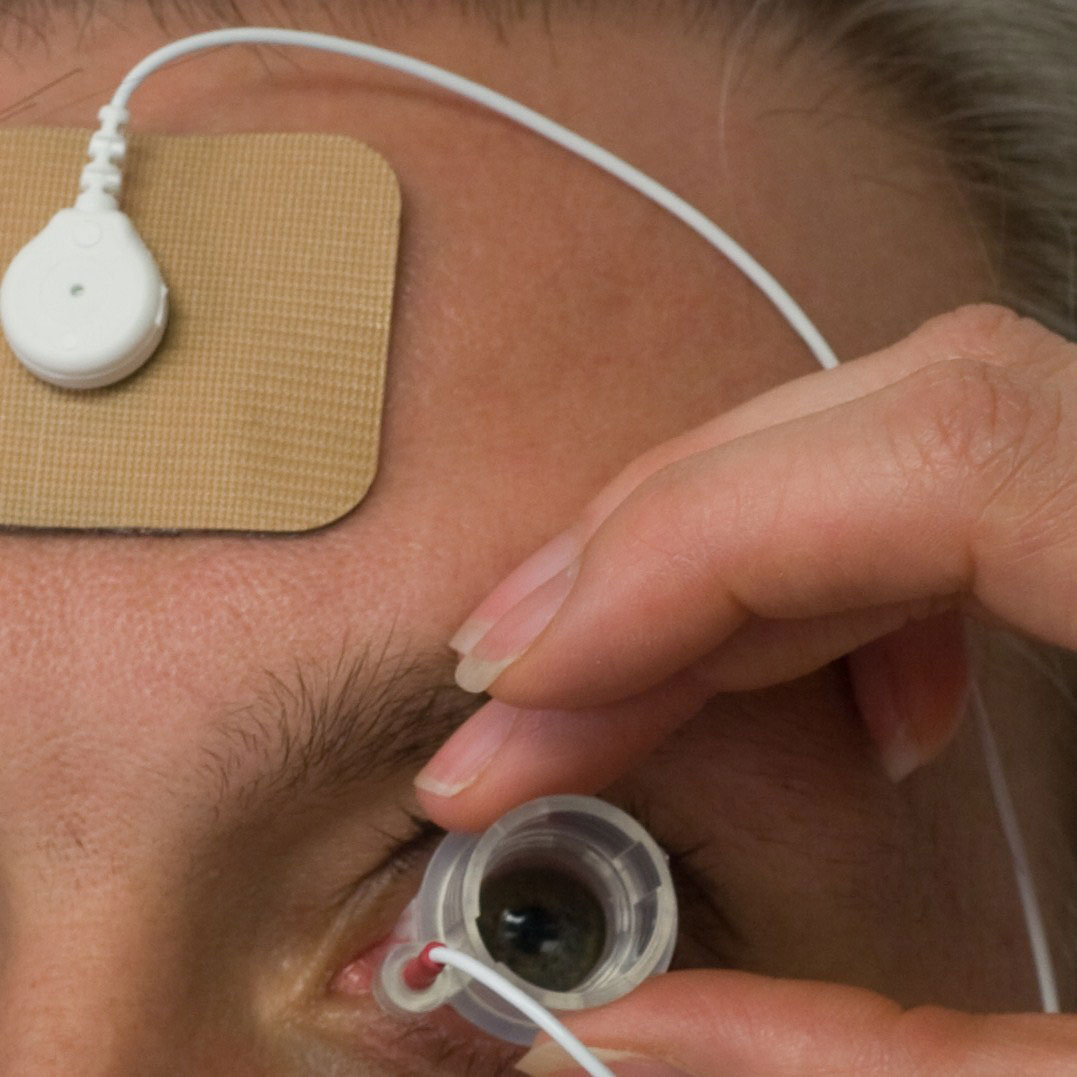 |
| The drug residing in the applicator is propelled through the conjunctiva and sclera during electrical stimulation. |
EyeGate researchers also looked into iontophoresis for anterior uveitis to establish safe, effective dose levels of EGP-437 and evaluate the systemic pharmacokinetic profile after a single iontophoresis administration of EGP-437 to 40 subjects at four different doses.9 The team found that following a single treatment with EGP-437, half of the patients achieved an anterior chamber cell count of zero within two weeks and the majority by day 28, requiring no further treatment.9 IOP and best-corrected visual acuities were not affected, and there was actually a lower incidence of increased IOP.9 While patients experienced low, short-term systemic exposure to dexamethasone following treatment, the researchers did not observe any corticosteroid mediated effects and add that the majority of AEs were mild.9
To date, roughly 2,000 EGP-437 iontophoretic treatments have been delivered to patients with anterior segment inflammation, posterior retinal edema or both, demonstrating the safety and utility of this product.
More recently, a team of researchers conducted the first-in-human, randomized, double-masked, dose-escalating study of iontophoretic administration of DP for scleritis, suggesting the iontophoretic delivery of corticosteroids to be a promising, well-tolerated and safe potential treatment.10 They found the lowest dose (1.2mA/minute at 0.4mA) to be the most efficacious, with five out of seven eyes meeting the primary efficacy outcome within 28 days.10
Commercialization Nears
After more than a decade of R&D, some of these efforts are approaching commercialization. EyeGate completed the second Phase III study in mid-2018 looking at the application of the EGP-437 in anterior uveitis and is continuing to assess the next steps toward approval. Once clinicians have access to the EyeGate II system, the eye care community will be able to learn more about its real-world performance and, ultimately, where it fits in the overall treatment armamentarium.
Dr. Wirostko is the chief medical officer of EyeGate Pharmaceuticals and an adjunct professor at the University of Utah. She practices at the John A. Moran Eye Center at the University of Utah.
Dr. Raizman is a consultant for the Ophthalmic Consultants of Boston, the director of the Cornea Fellowship and the Cornea and Cataract Service at the New England Eye Center, Tufts Medical Center and a professor at the Tufts University School of Medicine. He was the former chief medical officer of EyeGate Pharmaceuticals.
|
1. Novack GD. Ophthalmic drug delivery: development and regulatory considerations. Clin Pharmacol Ther. 2009;85(5):539-43. 2. Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005;57(14):2063-79. 3. Than TP, Chaglasian EL. Ocular drug delivery—pressing forward. RCCL. 2013;150(7):12-3. 4. Eljarrat-Binstock E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. J Control Release. 2006;110(3):479-89. 5. EyeGate Pharma. www.eyegatepharma.com. Accessed January 10, 2019. 6. Patane MA, Cohen A, From S, et al. Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: results of a randomized clinical trial. Clin Ophthalmol. 2011;2011(5):633-43. 7. Patane MA, Schubert W, Sanford T, et al. Evaluation of ocular and general safety following repeated dosing of dexamethasone phosphate delivered by transscleral iontophoresis in rabbits. J Ocul Pharmacol Ther. 2013;29(8). 8. Wirostko BM, Assang CM, Mann B, et al. Efficacy and safety of an iontophoresis platform to manage post-cataract inflammation and pain. Poster. ARVO. 2017. 9. Patane MA, Cohen A, Assang CM, et al. Randomized, double-masked study of EGP-437 in subjects with non-infectious anterior segment uveitis. Poster. AAO. 2010. 10. O’Neil EC, Huang J, Suhler EB, et al. Iontophoretic delivery of dexamethasone phosphate for non-infectious, non-necrotising anterior scleritis, dose-finding clinical trial. Br J Ophthalmol. 2018;102(8):1011-3. |

