
Suppose you have a patient with herpes simplex keratitis who experiences severe corneal toxicity secondary to Viroptic (trifluridine 1%, GlaxoSmithKline) or who requires antiviral coverage during the night. Traditionally, we prescribed Vira-A (vidarabine 3%) ointment, but this product is no longer manufactured.
This is one of many situations in which you might use a compounding pharmacy, which could prepare the ointment for your patient. This article offers examples of how patients might benefit from compounded therapeutics.
Customized Medication
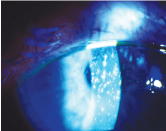
Acetylcysteine can be compounded and used off-label for such conditions as filamentary keratitis or filaments associated with superior limbic keratoconjunctivitis, seen here.
Compounding is defined as customized medication prepared by a pharmacist according to a doctor"s specifications to meet an individual patients need.
According to Lee Pharmacy in
In recent years, however, doctors and patients have again come to appreciate the advantages of customized medications. In a four-state survey conducted in 2006, 94% of the 370 pharmacists responded that they provide at least some compounding services.1 Additionally, a nationwide study found that 92% of surveyed pharmacies reported compounding capabilities.2 Compounding at many locations may be limited to topical preparations, although more full-service compounding pharmacies can provide oral and parenteral formulations.
Today, an estimated 30 million to 40 million prescriptions are compounded each year.3 Twenty-five years from now, technology will have propelled pharmacists back to the future of compounding and personalized care, says Jerome Halperin, former chief executive of the United States Pharmacopeia (USP).
Additional Resources About Compounding Pharmacies Compounding Pharmacy Safety Checklist: www.apothecure.com/pdfs/safety-checklist.pdf Professional Compounding Centers of America, Inc. (PCCA) 1-800-331-2498 1-800-927-4227 Center for Drug Evaluation and Research Patients and Professionals for Customized Care (P2C2)
Why Compound?
Compounding pharmacies are located throughout the country and may have varying degrees of familiarity with ophthalmic products. Compounding pharmacies are licensed and regulated by their respective state boards and must follow strict USP guidelines for compounding. These guidelines help ensure that compounded sterile preparations are of the highest quality. A voluntary Pharmacy Compounding Accreditation Board (PCAB) conducts annual reviews and site visits every three years. To find an accredited compounding pharmacy in your area, consult www.pcab.org. (To obtain a checklist of questions about safety and other resources, see Additional Resources About Compounding Pharmacies, right.)
The main reason for compounding prescriptions is the unavailability of a commercial product. Compounding pharmacies can also:
Prepare medications that are not commercially available, are not economically feasible to manufacture, or are not stable.
Prepare formulas for individual needs with a special strength.
Add flavor to make an oral medication more palatable.
Prepare unique routes of administration, such as a quick-dissolving lozenge, a lollipop or a transdermal patch.
Dermatological preparations are one of the largest categories of compounded medications. Many drugs are compounded for pediatric use, as commercially available drugs often are not available at appropriate concentrations or routes.
Combating Drug Resistance
Although ophthalmic medications do not represent a significant amount of compounded medications, there are many circumstances in which an ocular condition is best treated with a compounded therapeutic agent.
Anatomy of an Rx When writing a prescription for a drug to be prepared by a compounding pharmacy, the first line on the prescription should begin with Compounded Medication. Information should specifically include the generic name of the active ingredient, strength or dose, dosage form (e.g., drop or gel), quantity, directions for use and indication.
Although the fourth-generation fluoroquinolones offer greater broad-spectrum coverage with improved efficacy against gram-positive organisms than earlier generation agents, the emergence of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has proven in some cases to be non-susceptible to fluoroquinolones. Data that was voluntarily reported to The Surveillance Network (TSN) showed that the proportion of S. aureus ocular infections that were culture-positive for MRSA increased from 29.5% in 2000 to 41.6% in 2005.4 The MRSA ocular isolates were resistant to three or more antibiotics, including fluoroquinolones. These findings suggest MRSA ocular infections may become more prevalent than methicillin-susceptible S. aureus (MSSA) within the next few years.4
The Ocular Tracking Resistance in
CA-MRSA most often manifests as skin and soft tissue infections, including lid infections, preseptal cellulitis and dacryocystitis. Conjunctivitis is the most commonly reported ocular involvement from CA-MRSA, although this organism may also be isolated in corneal ulcers, blebitis and endogenous endophthalmitis.6
CA-MRSA ocular infections that require topical treatment, such as conjunctivitis, can be managed with vancomycin drops. This requires the services of a compounding pharmacy that can prepare a 50mg/ml concentration of vancomycin, which is stable at room temperature for two weeks. Chloramphenicol eye drops, while effective, are often avoided due to the risk of development of aplastic anemia. (However, two international studies a decade ago found no data to support this claim.7) Other examples of fortified topical antibiotics include cefazolin, commonly prescribed as 50mg/ml; gentamicin, commonly 14mg/ml; and tobramycin, commonly 14mg/ml.
Hold the Preservatives

A combination of dilating/cycloplegic agents, such as tropicamide 0.5%, cyclopentolate 0.5% and phenylephrine 2.5%, in spray formulation can be applied to closed eyelids.
Patients who are sensitive to all preservatives pose a significant challenge, even when they simply present for a comprehensive eye examination. A compounding pharmacy can prepare preservative-free diagnostic agents.
Combining dilating/cycloplegic agents, such as tropicamide 0.5%, cyclopentolate 0.5%, and phenylephrine 2.5%, in spray formulation that can be applied to closed eyelids proves invaluable in the pediatric population.
Hydroxyamphetamine hydrobromide, although no longer commercially available as Paredrine, is an important agent in differentiating central vs. peripheral Horners pupil and can be obtained from a compounding pharmacy.
One precaution: Preservative-free agents have a relatively short shelf life. If you anticipate limited use of such medications, consider ordering a very small quantity, such as 1ml.
Patients who are sensitive to preservatives pose treatment challenges when they need chronic topical ocular therapy. Some examples:
Anti-glaucoma agents. In a multicenter study of more than 9,000 patients on topical beta-blockers, the researchers noted significantly less ocular irritation, both subjectively and objectively, in patients who used preservative-free (PF) topical beta-blockers vs. those who used the preserved versions.8 If ocular symptoms from chronic use of preserved anti-glaucoma agents impacts adherence, patients may benefit from compounded PF agents, such as dorzolamide, latanaprost or brimonidine.
Anti-allergy agents. In a study comparing use of preserved and PF anti-allergy medications, patients who used PF formulations experienced statistically significant improvement in comfort.9 PF eye drops result in better tear stability, better corneal barrier function, lower conjunctival cell apoptosis and less toxicity.10 So, you might consider having a compounding pharmacy prepare PF drugs, such as sodium chloride 5% or cromolyn sodium 4% for those patients who require chronic treatment.
Mycolytic agents. For example, 10% acetylcysteine can be compounded and used off-label for such conditions as filamentary keratitis or filaments associated with superior limbic keratoconjunctivitis. These drops are typically used q.i.d. in the affected eye(s).
Off-Label Use
This last example brings up an important issue: off-label use. Of the 725 million prescriptions written for the 500 most commonly used drugs, 21% of the prescriptions are for off-label uses.13 Requests for compounded drugs for ophthalmic applications are often for off-label applications.
Once a drug has satisfied safety and efficacy tests for a specific use and receives FDA approval, that particular indication is considered on-label use. Once a drug is approved, physicians often prescribe it for different conditions, different populations or in different dosesall of which constitute off-label use.11
Off-label use is generally legal, but cannot be promoted by the drug manufacturer. The FDA explicitly states that once a [pharmaceutical] product has been approved for marketing, a physician may prescribe it for uses in treatment regimes of patient populations that are not included in the approved labeling.12
It is expensive and time-consuming for a pharmaceutical company to obtain additional on-label drug indications. Trials can take seven to 10 years and cost millions of dollars. Motivation to obtain additional indications is even less once a drug becomes generic. Off-label uses allow lower costs for new therapies, permit treatment alternatives when on-label indications fail, and allow for discovery of new uses for existing drugs.
Seventy-five percent of prescription drugs have no indications for children, so off-label use is often necessary. Pregnant women and nursing mothers are often not included in drug studies, so prescriptions are usually off-label when treating these populations.
Prescribing a commercially available medication or requesting a compounded drug for off-label use is appropriate if sufficient scientific rationale indicates the drug may be effective for that purpose. You should be well informed about the drug prior to prescribing. A main consideration is whether that prescription meets the standard of practice among eye care providers.
Reimbursement for medications used off-label varies. Medicare Part D covers off-label prescriptions only if the particular use is documented in one of three medical compendia.13 There is no standardized method of processing a claim for a compounded medication but the pharmacist can assist the patient.
Drugs that are manufactured are assigned National Drug Code numbers (NDC), which are unique three-segment numbers that standardize the reimbursement process. A compounded drug does not receive an NDC number; however, reimbursement should be based on the prescription from a licensed practitioner, not whether the drug has an NDC number.
The use of compounded drugs can provide better and individualized care as we manage patients with ocular disease. A compounding pharmacist can provide additional agents for your diagnostic and treatment arsenal.
Dr. Than is an associate professor at
1. McPherson TB,
2. Treadway AK, Craddock D, Leff R. Practices of pharmacies that compound extemporaneous formulations. Am J Health Syst Pharm 2007 Jul 1;64(13):1403-9.
3. The
4. Asbell PA, Sahm DF, Shaw M, et al. Increasing prevalence
of methicillin resistance in serious ocular infections caused by Staphylococcus aureus in the
5. Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationside antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol 2008 Jun;145(6):951-8.
6. Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2006;104:322-45.
7. Wiholm B, Kelly JP, Kaufman D, et al. Relation of aplastic anemia to use of chloramphenicol eye drops in two international case-control studies. BMJ 1998 Feb 28;316(7132): 666.
8. Jaenen N, Baudouin C, Pouliquen P, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol 200 May-Jun;17(3): 341-9.
9. Beden C, Helleboid L, Marmouz F,
10. Baudouin C. Allergic reaction to topical eyedrops. Curr Opin Allergy Clin Immunol 2005 Oct;5(5):459-63.
11. Fugh-Berman A, Melnick D. Off-label promotion, on-target sales. PLoS Med 2008 Oct 28;5(10):e210.
12. Wilkes M, Johns M. Informed consent and shared decision-making: a requirement to disclose to patients off-label prescriptions. PLoS Medicine 2008 Nov 11;5(11):e223.
13.

