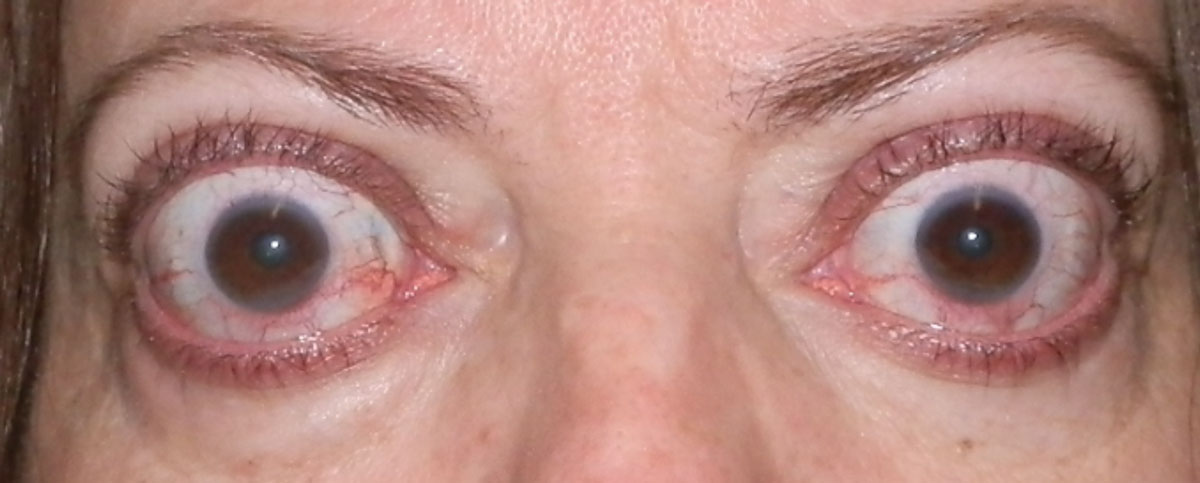
 |
Some patients treated with teprotumumab (Tepezza) for TED may experience hearing loss as an adverse effect. Photo: Bobby Saenz, OD, MS. Click image to enlarge. |
Researchers recently reported hearing-related side effects with teprotumumab use in a small case series of 27 patients, recommending audiometry with patulous eustachian tube (PET) testing at baseline.
In the prospective case series, 81.5% of patients developed new subjective otologic symptoms after an average of 3.8 infusions of teprotumumab. At follow-up after the final infusion, 100% of patients with tinnitus, 90.9% with ear plugging/fullness, 83.3% with autophony and 45.5% with subjective hearing loss or decreased word comprehension had symptom resolution.
The researchers noted that, of the six patients who underwent baseline and post-treatment audiometry, five developed teprotumumab-related sensorineural hearing loss (three of whom had persistent subject hearing loss at final follow-up) and one developed PET. The researchers reported that history of hearing loss was a risk factor for the drug-related hearing loss. Though most patients weren’t functionally impacted by their symptoms, four wore hearing aids during the study and three discontinued therapy.
“Hearing dysfunction has been described as a side effect in 10% of patients in the randomized clinical trials [of teprotumumab],” the researcher wrote in their paper. “Our results demonstrate a much higher percentage of teprotumumab-treated thyroid eye disease patients with new otologic symptoms than previously reported […]. Patients with baseline hearing loss should be counseled on this potential risk and strongly advised to undergo baseline audiometric testing with frequent monitoring.”
Sears CM, Azad AD, Amarikwa L, et al. Hearing dysfunction after treatment with teprotumumab for thyroid eye disease. Am J Ophthalmol. February 25, 2022. [Epub ahead of print]. |

