Glaucomatocyclitic crisis (GCC), or Posner-Schlossman syndrome, was first described by Posner and Schlossman in 1948 as a rare, recurrent and typically unilateral inflammatory ocular hypertensive disease.1 It generally affects one eye at a time, and its recurrence usually afflicts the same eye. Bilateral and simultaneous involvement is very uncommon. The individual attacks may last from a few hours to a few weeks, but rarely persist over two weeks. Episodes may occur with varying frequency and without any apparent cause. It can affect adults of all ages (reports range from 23 to 67 years), especially between the third and sixth decade of life.2,3 Only two pediatric cases of GCC have been reported.2,4 One study looking at population statistics in Finland found the prevalence to be 1.9 per 100,000.5 No racial or sexual predilection has been reported.
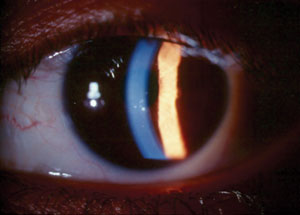 | |
| Fig. 1. Small, centrally located keratic precipitates in an eye with glaucomatocyclitic crisis. |
The classical presentation of GCC is a unilateral ocular hypertension with mild anterior segment inflammation (cyclitis) and few clinical symptoms. The elevation of intraocular pressure (IOP), which can range from 40mm Hg to 70mm Hg or above, is out of proportion with the minimal symptoms reported by the patient and is characteristic of this inflammatory disease. Recurrent episodes further help confirm the diagnosis. During intervals between attacks, the patient is asymptomatic.
Pathophysiology
The cause and pathogenesis of GCC remains poorly understood, in part because the mild anterior chamber reaction noted during attacks does not correlate with the magnitude of IOP elevation, as opposed to what is usually seen in uveitic glaucoma. It is therefore believed that the locus of the inflammation is the trabecular meshwork, leading to decreased aqueous outflow, which in turn leads to elevated IOP. However, it is not yet clear what causes the inflammation and whether the mechanism is infectious in nature or strictly inflammatory.
Using polymerase chain reaction analysis, studies investigating aqueous humor composition during acute attacks of GCC have not found a conclusive causative factor. In one study, examination of the aqueous humor identified the presence of cytomegalovirus (CMV) but not other herpes viruses.6 Another study found herpes simplex virus (HSV) within the aqueous while testing negative for varicella zoster virus (VZV) or CMV.7 Yet more studies that analyzed the contents of the aqueous humor found that, during the acute phase, there is a measurable increase in the aqueous levels of prostaglandins, particularly prostaglandin E, which levels have been found to positively correlate with IOP.7 During periods of remission, the study also demonstrated these same levels were markedly decreased with concurrent increased aqueous outflow.
A likely hypothesis about the exact relationship between prostaglandin induction and increased IOP is a dual mechanism characterized by the stimulation of aqueous production as well as the inhibition of its outflow. The outflow inhibition caused by breakdown of the blood-aqueous barrier, leading to the release of inflammatory cells, which results in clogging of the trabecular meshwork.8,9 However, it has not yet been shown whether the presence of prostaglandin is causative of the disease or simply an epiphenomenon of an underlying mechanism.
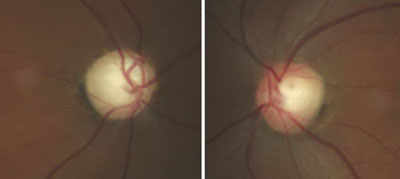 | |
| Fig. 2. Glaucomatous optic nerve damage in the right eye of a patient with GCC and repeated IOP elevations exceeding 55mm Hg. |
A recent article analyzing the different aqueous humor composition studies argued that the most reliable and reproducible studies—taking into consideration the sample size and whether the study included testing for other particles—were those that found CMV had the greatest association with GCC.10 However, the exact mechanism of GCC, as well as whether the presence of viral particles is causative of the disease or is indirectly related to it through an unknown mechanism, is still unclear.11
Clinical Presentation
Patients typically present during active episodes of GCC with symptoms ranging from slight orbital or ocular discomfort to colored halos or blurred vision resulting from corneal edema caused by elevated IOP. In cases where IOP is very high, significant decrease in visual acuity due to corneal edema and pain may be present.2
Clinical findings often include markedly elevated IOP, open anterior chamber angles seen by gonioscopy, mild cyclitis presenting as rare aqueous inflammatory cells and a few small keratic precipitates (Figure 1). IOP may increase to as much as 70mm Hg or more in some cases. Conjunctival hyperemia is usually absent unless IOP is very high, in which case mild congestion may be noted. Given the small degree of anterior chamber inflammatory reaction, posterior synechiae are not typically present. Similarly, peripheral anterior synechiae are also absent. Heterochromia can be present where the affected eye is light-colored due to either sectoral or diffuse iris atrophy resulting from prolonged IOP elevation and tissue ischemia.
In general, optic discs are normal and no visual defects are noted, especially with initial episodes of GCC. However, repeated or prolonged episodes can cause typical glaucomatous damage. Additionally, two cases of non-arteritic anterior ischemic optic neuropathy (NAAION) associated with GCC attack have been published.12,13 One postulated that GCC-induced elevated IOP, along with risk factors such as systemic conditions (e.g., hypertension and diabetes) and a “disc at risk” (i.e., crowded disc and minimal physiological cup), contributed to a decreased ocular disc perfusion, leading to vision loss and subsequent optic nerve atrophy.12
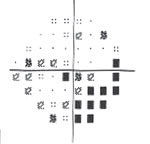 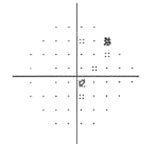 | |
| Fig. 3. Visual field testing reveals glaucomatous field loss OD. |
The original description of GCC by Posner and Schlossman stated that during intervals between attacks, no sign of inflammation is present, and IOP is generally normal.1 However, some patients may show slightly elevated pressure in both the affected and unaffected eye.1 One study that followed patients with GCC between attacks identified a high incidence of elevated IOP, decreased facility of aqueous outflow, higher IOP response to topical steroids, glaucomatous disc damage and visual field loss in the non-affected and GCC-involved eye.14 These observations indicate that patients with GCC are at a greater risk of developing primary open angle glaucoma in either eye.14
Differential Diagnoses
Several diseases can be easily confused with GCC. These include:
- Acute Angle-closure Glaucoma. This condition presents with symptoms of unilateral pain, redness, blurry vision, headaches, nausea and vomiting. Clinical signs include a closed angle seen by gonioscopy, peripheral anterior synechiae, corneal edema and markedly increased IOP. Glaucomatous damage typically ensues quickly if the attack is not managed; this is considered an ocular emergency.
- Uveitic Glaucoma. This condition has elevated IOP often associated with a significant anterior chamber reaction, which is not present in GCC.
- Fuchs’ Heterochromic Iridocyclitis. This condition is a chronic mild cyclitis usually associated with heterochromia. Similar to GCC, it is unilateral with an absence of acute symptoms. However, the increase in IOP that results from this disease is rarely as high as seen in GCC. Fuchs’ heterochromic iridocyclitis is associated with cataract formation and does not respond to anti-inflammatory agents.
- Herpetic Keratouveitis. This condition clinically presents as a painful, red eye—in contrast to the less inflamed eye often seen with GCC—with elevated IOP and mild anterior segment inflammation. Patients often have a history of a previous ocular herpetic infection.
When presenting as a primary infection, herpetic keratouveitis rarely comes in the form of trabeculitis or cyclitis; however, lid lesions, follicular conjunctivitis or epithelial corneal dendrites are commonly found. Reactivation of the latent virus after primary infection can lead to more severe ocular complications, including stromal keratitis, trabeculitis, uveitis and endothelialitis.14 When trabeculitis presents alone, possible features that can help in the differential diagnosis are the presence of eyelid or epithelial scars and loss of corneal sensitivity from a previous primary infection.
Management
Glaucomatocyclitic crisis is generally considered a self-limited condition because the inflammation and subsequently elevated IOP will subside by itself within a few days to a few weeks. However, when it occurs, exceptionally high IOP during the crisis can cause permanent nerve damage if left untreated. Also, due to the recurrent nature of the disease, the repetitious IOP elevation can result in glaucomatous vision loss over time (Figures 2 and 3).
Given that the mechanism of the disease is primarily inflammatory, the management of GCC largely includes treating the underlying inflammation with a short course of topical steroids. Additionally, the initial management includes lowering IOP with topical anti-glaucomatous medications. Successful management with β-blockers, α-agonists, carbonic anhydrase inhibitors (CAIs) or a combination of the three, has been reported.16,17 Prostaglandin analogs and miotics should be generally avoided since these agents are known to potentially increase inflammation. Anti-inflammatory and anti-glaucoma medications should be maintained until complete resolution of the attack. Second-line therapy may include oral CAIs to manage the elevated IOP as well as topical NSAIDs, oral NSAIDs or both, to control the inflammation.
In patients with multiple recurrences or prolonged attacks, more definitive surgery has been proposed to fully control IOP while avoiding the complications of long-term topical steroid use. Trabeculectomy has been shown to be a safe and effective method in GCC patients and should be considered as soon as they start showing glaucomatous changes, since progression can be quite rapid in these patients.3,14,18,19 As a filtering procedure, it provides an alternative pathway for aqueous outflow and thus prevents spikes during episodes of cyclitis.3
Long-term management should include periodic observation in order to detect a possible conversion to open angle glaucoma in either eye, which has a higher incidence among GCC patients. The risk of developing open angle glaucoma is directly related to the number and duration of GCC attacks.3 Patients who present with longer duration attacks should be monitored more carefully.
No definitive treatment has been reported for prevention of GCC recurrences. Given the strong association with CMV, investigators have tried some promising antiviral treatments. Thus far, topical ganciclovir 0.15% has demonstrated the greatest potential in decreasing the recurrence of GCC attacks with the least amount of side effects.11 One study demonstrated topical ganciclovir was effective for clearing the viral load, assisting in IOP control and preserving the corneal endothelium of CMV-positive GCC patients.20 Researchers surmised topical ganciclovir could assist in the management of patients with GCC, possibly decreasing the risk of advanced glaucoma and helping avoid glaucoma surgery in long-lasting cases.20
Another study indicated that long-term oral therapy with Valcyte (valganciclovir, Hoffmann–La Roche) 900mg BID for three weeks, followed by 450mg BID for a mean period of 20 months, lowered the recurrence rate in patients with GCC.21 Two of the 11 patients studied had recurrence once the medication was stopped, but the others remained disease-free.21 More studies are needed to test the efficacy and side effects resulting from the use of these drugs for extended periods.
While GCC typically presents with few, if any, symptoms, there are exceptions where the patient may present in acute distress. Considering GCC in the differential diagnosis in the presence of an acute unilateral elevated IOP is important, as proper management and treatment can prevent vision loss in some cases. Taking a detailed history and evaluating the anterior segment, including gonioscopy, plays a key role in the correct diagnosis. Additionally, while GCC is sometimes considered a benign, self-limiting condition, it is clear that some patients experiencing recurrent attacks can develop glaucomatous vision loss.
Patient education should stress the recurrent nature of the disease and the potential for visual compromise, as well as the importance of regular follow ups even between the attacks.
Dr. Makhlouf is an assistant professor with a focus on primary eye care and ocular disease at Nova Southeastern University College of Optometry in Fort Lauderdale, Fla. She teaches the course in ophthalmic lasers and surgical co-management.
Dr. Sowka is chief of Advanced Care and director of the Glaucoma Service at Nova Southeastern University College of Optometry. He is the lead author of The Handbook of Ocular Disease Management, published annually by Review of Optometry.
1. Posner A, Schlossman A. Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Arch Ophthal. 1948;39:517-35.2. Burnstein Y, Shelton K, Higginbotham EJ. Glaucomatocyclitic crisis in a child. Am J Ophthalmol. 1998;126:136-7.
3. Jap A, Sivakumar M, Chee SP. Is Posner Schlossman syndrome benign? Ophthalmology. 2001;108:913-18.
4. Teekhasaenee C, Ritch R. Glaucomatocyclitic crisis in a child. Am Journal Ophthalmol. 1999;127:626-7.
5. Paivonsalo-Hietanen T, Tuominen J, Vaahtoranta-Lehtonen H, et al. Incidence and prevalence of different uveitis entities in Finland. Acta Ophthalmol Scand. 1997;75(1):76-81.
6. Bloch-Michel E, Dussaix E, Cerqueti P, Patarin D. Possible role of cytomegalovirus infection in the etiology of the Posner-Schlossmann syndrome. Int Ophthalmol. 1987;11:95-6.
7. Yamamoto S, Pavan-Lagston D, Tada R, et al. Possible role of herpes simplex virus in the origin of Posner-Schlossman syndrome. Am J Ophthalmol.1995;119:796-8.
8. Eakins KE. Increased intraocular pressure produced by prostaglandins E1 and E2 in the cat eye. Exp Eye Res. 1970;10:87-92.
9. Grant WM. Clinical measurements of aqueous outflow. Am J Ophthalmol. 1951;34:1603-5.
10. Takusagawa HL, Liu Y, Wiggs JL. Infectious theories of posner-schlossman syndrome. International Ophthalmology Clinics. 2011;51:105-15.
11. Teoh SB, Thean L, Koay E. Cytomegalovirus in aetiology of Posner-Schlossman syndrome: evidence from quantitative polymerase chain reaction. Eye. 2005;19:1338-40.
12. Irak I, Katz BJ, Zabriskie NA, et al. Posner-Schlossman syndrome and nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol., 2003;23:264-7.
13. Kim R, Van Stavern G, Juzych M. Nonarteritic anterior ischemic optic neuropathy associated with acute glaucoma secondary to Posner-Schlossman syndrome. Archives Ophthalmol, 2003;121:127-28.
14. Kass MA, Becker B, Kolker AE. Glaucomatocyclitic crisis and primary open-angle glaucoma. Am J Ophthalmol.1973;75:668-73.
15. Saini JS, Agarwala R. Clinical pattern of recurrent herpes simplex keratitis. Indian J Ophthalmol. 1999;47:11-4.
16. Muthusamy P. Apraclonidine in the management of glaucomatocyclitic crisis. Eye (London, England); 1994;8: 367-368.
17. Hong C, Song KY. Effect of apraclonidine hydrochloride on the attack of Posner-Schlossman syndrome. KJO, 1993;7:28-33.
18. Dinakaran S, Kayarkar V. Trabeculectomy in the management of Posner-Schlossman syndrome. Ophthalmic Surg Lasers. 2002;33:321-22..
19. Zhong Y, Cheng Y, Liu X, Feng P. Trabeculectomy in the management of glaucomatocyclitic crisis with visual field defect. Ocul Immunol Inflamm. 2012;18:233-36.
20. Su CC, Hu FR, Wang TH, et al. Clinical outcomes in cytomegalovirus-positive Posner-Schlossman syndrome patients treated with topical ganciclovir therapy. Am J Ophthalmol. 2014;158(5):1024-3.
21. Sobolewska B, Deuter C, Doycheva D, Zierhut M. Long-term oral therapy with valganciclovir in patients with Posner-Schlossman syndrome. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):117-24.

