 An 81-year-old white female presented with visual field changes that were suggestive of a conversion to glaucoma. Her chief complaint was of a gradual, progressive decrease in vision that began six months earlier. Her vision loss was worse in her left eye than her right eye.
An 81-year-old white female presented with visual field changes that were suggestive of a conversion to glaucoma. Her chief complaint was of a gradual, progressive decrease in vision that began six months earlier. Her vision loss was worse in her left eye than her right eye.
Her ocular history was significant for bilateral cataract surgery in fall of 2008. Additionally, we had been monitoring her as a glaucoma suspect for several years.
Her current medications included Restasis (cyclosporine, Allergan) b.i.d., O.U., atenolol, amiodarone, Coumadin (warfarin sodium, Bristol-Myers Squibb), Lipitor (atorvastatin calcium, Pfizer), hydrochlorothiazide and vitamin K supplementation. She reported no known allergies.
Diagnostic Data
Prior to cataract surgery, her best-corrected visual acuity was 20/50 O.D. and 20/70 O.S. Her preoperative potential acuity meter (PAM) measurements were 20/25 O.D. and 20/30 O.S. Her acuity fluctuated depending on corneal integrity and tear film quality, but decreased secondary to cataract progression.
Prior to cataract surgery, we followed the patient for dry eye disease, dry age-related macular degeneration, posterior vitreous detachments and ocular hypertension. Both corneas had been characterized by variable amounts of diffuse superficial punctate keratopathy on the inferior aspect of the corneas. She also had bilateral scant tear prisms and decreased tear film break-up times. Her lid margins were characterized by mild lid wiper epitheliopathy and a chronic, low-grade marginal blepharitis. And, she exhibited bilateral dermatochalasis.
Preoperative IOP averaged 21mm Hg O.S. and 23mm Hg O.S. Pachymetry readings measured 557µm O.D. and 548µm O.S. Gonioscopic examination revealed grade 2 to grade 3 open angles 360˚ O.U., with grade 1 to grade 2 trabecular pigmentation O.U. She had no angle abnormalities.
Threshold visual fields were essentially unremarkable, except for a global depression that was attributable to her cataracts. Heidelberg Retina Tomography-3 (HRT-3, Heidelberg Engineering) scans of both optic nerves revealed no significant neuroretinal rim abnormalities and remained unchanged from earlier scans. Her optic nerves were characterized by 0.50 x 0.55 cupping O.U. Both maculae were characterized by retinal pigment epithelium (RPE) granulation and window defects centered in the foveal avascular zone (FAZ), as well as drusen scattered in both maculae (O.S.>O.D.). Her peripheral retinal evaluations were unremarkable.
Also, her crystalline lenses were characterized by grade 2 to grade 3 nuclear cataracts O.U., with cortical spoke encroaching on the visual axis.
Three months after cataract surgery, her best-corrected visual acuity improved to 20/30- O.D. and 20/40 O.S. Her pupils were equal, round and reactive to light and accommodation, with no afferent pupillary defect. Her IOLs were well centered in the capsular bags, and the posterior capsules were clear and intact.
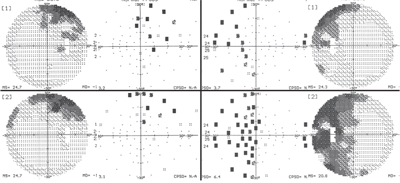
Our
patient’s visual field results (O.D. left, O.S., right) at her June
(top images) and September 2009 (bottom images) visits. Note the
significant progression in her left eye.
Evaluation of her maculae revealed the presence of epiretinal membranes. (The right macula was centered along the superior arcades and the left macula involved the FAZ.)
In June 2009, the patient presented for a scheduled ocular hypertension visit. At this visit, her IOP measured 22mm Hg O.D. and 24mm Hg O.S. She complained of redness and irritation in both eyes (O.S.>O.D.) that had persisted for several weeks. She reported that the increased use of artificial tears provided no relief. She also noted “blurry” vision in her left eye.
Pinhole acuity was 20/40 O.D. and 20/60 O.S. Visual fields suggested the early development of a superior arcuate defect; but, this finding did not correlate with her optic nerve appearance. In fact, her visual fields seemed more consistent with dermatochalasis than glaucoma.
Both corneas demonstrated staining patterns that were consistent with dry eye disease (O.S.>O.D.). I diagnosed her with mild episcleritis O.U. secondary to chronic dry eye. I wrote her a prescription for Zylet (loteprednol etabonate 0.5% and tobramycin 0.3%, Bausch & Lomb) q.i.d. O.U.
In September 2009, she presented for a follow-up and reported that while both eyes were still comfortable, the vision in her left eye had worsened. Her visual acuity measured 20/30 O.D. and 20/200 O.S. Also, she exhibited no changes to her optic nerves, maculae, peripheral retinae, IOLs, capsules or corneas.
However, her visual fields showed dramatic changes (O.S.>O.D.).
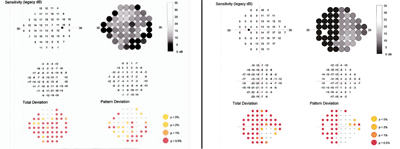
Our patient’s HEP results (O.D. left, O.S. right) show neurological field defects.
Discussion
At the September 2009 visit, her dry eye was relatively well controlled and could not have accounted for such a marked change in her visual field. Also, we were fairly certain that her visual field change was not a result of a conversion to glaucoma. We noted no structural changes to her optic nerves on HRT-3 to correlate with her visual fields, and her left visual field was striking in its general adherence to the left hemifield. It is possible, however, that the visual fields in her left eye were glaucomatous in origin, if we were to interpret them as an early above and below arcuate defect. But, it would be quite rare for such an above and below arcuate defect to not cross the vertical neurological midline.
This observation raises the point that this field defect could be neurological. While the left field defect “hugs” the vertical midline, it does not clearly respect it. And, if this defect were neurological in nature, it would be, by necessity, bilateral, implying chiasmal or post-chiasmal origin.
Close examination of the right eye’s visual field demonstrated no left-sided hemifield defect; if present, this finding would be suggestive of a post-chiasmal visual pathway problem. Additionally, we saw no clear right-sided temporal hemifield defect either; if present, this finding would be suggestive of a chiasmal pathology. Nonetheless, there was some temporal aspect to the field defect in the patient’s right eye.
Clearly, there was a demonstrable change in both visual acuity and visual field in her left eye. These findings raised my level of suspicion for an occult pathology. But, did these findings warrant an MRI?
I asked her to return in a few days for another visual field study. This time, I used the Heidelberg Edge Perimeter (HEP, Heidelberg Engineering), which targets M-cells in the retina, and identifies field defects earlier than standard white-on-white perimetry. (See “ Perimetry Gets an Upgrade,” June 2009.)
Close examination of the HEP fields showed a neurological field defect. Her left eye exhibited a temporal field defect that respected the vertical midline. The right eye also showed a temporal defect; however, the defect was greater inferiorly than superiorly.
Based on the HEP fields and clinical suspicion, I ordered an MRI with emphasis on the chiasm. The MRI revealed a large pituitary adenoma that appeared very asymmetric. The bulk of the mass was on the left side, with only one small lobe affecting the right side of the chiasm. The asymmetry in the pituitary mass correlated well with the asymmetry in her bitemporal hemianopia.
This case highlights several important items. First, while we sometimes focus on creating a treatment plan for a problem that we have been following, it is important to be ever vigilant for occult disease. Also, early visual field defects associated with glaucoma often occur in the temporal fields adjacent to the physiological blind spot, as seen in this patient. It is also important to note that in some conditions, especially those with varying, non-classical symptoms or findings, it simply takes time and close monitoring for the problem to manifest.
Our patient underwent a transsphenoidal resection of her pituitary adenoma the week before Christmas 2009. Currently, she is doing well. A report on her postoperative fields, however, is still pending evaluation.

