With the continued move to medical models of care delivery and the increased scope of optometric practice across the country and the world, it is more important than ever that optometrists be well-educated on prescribing therapeutics for ocular disease.
There are many nuances to safe and effective prescribing of oral medications in adult and pediatric populations for infectious and inflammatory conditions. Maximizing treatment success requires a careful understanding of pathophysiology, individual patient characteristics, known patterns of resistance, pharmacokinetics, insurance coverage and availability of medications.
Here, I will address some of the most frequently asked prescribing questions in clinical practice and update you on the latest guidelines for treating various types of patients with a variety of oral medications.
1. Oral antiviral agents: are they all the same?
The shift in prescribing away from topical antiviral agents in the setting of herpes simplex epithelial keratitis to oral agents reflects the efficacy, safety and availability of these agents in the United States.1 In treating herpes zoster and herpes simplex ophthalmic infections, prescribers have options when it comes to choosing an oral antiviral agent. Famciclovir, acyclovir and valacyclovir are all available as branded and generic products, are all effective and all have an excellent margin of safety for the majority of individuals requiring treatment of ocular infections due to herpes simplex and varicella zoster virus.1-6 Common adverse effects include headache and nausea.1 All oral antiviral medications are cleared through the renal system, and renal dysfunction is known to be a rare but serious potential adverse effect. Individuals should be advised to drink plenty of water while undergoing therapy to reduce the likelihood of such adverse effects. In addition, individualized dosing adjustments must be made for individuals with reduced renal insufficiency or failure based on creatinine clearance.1
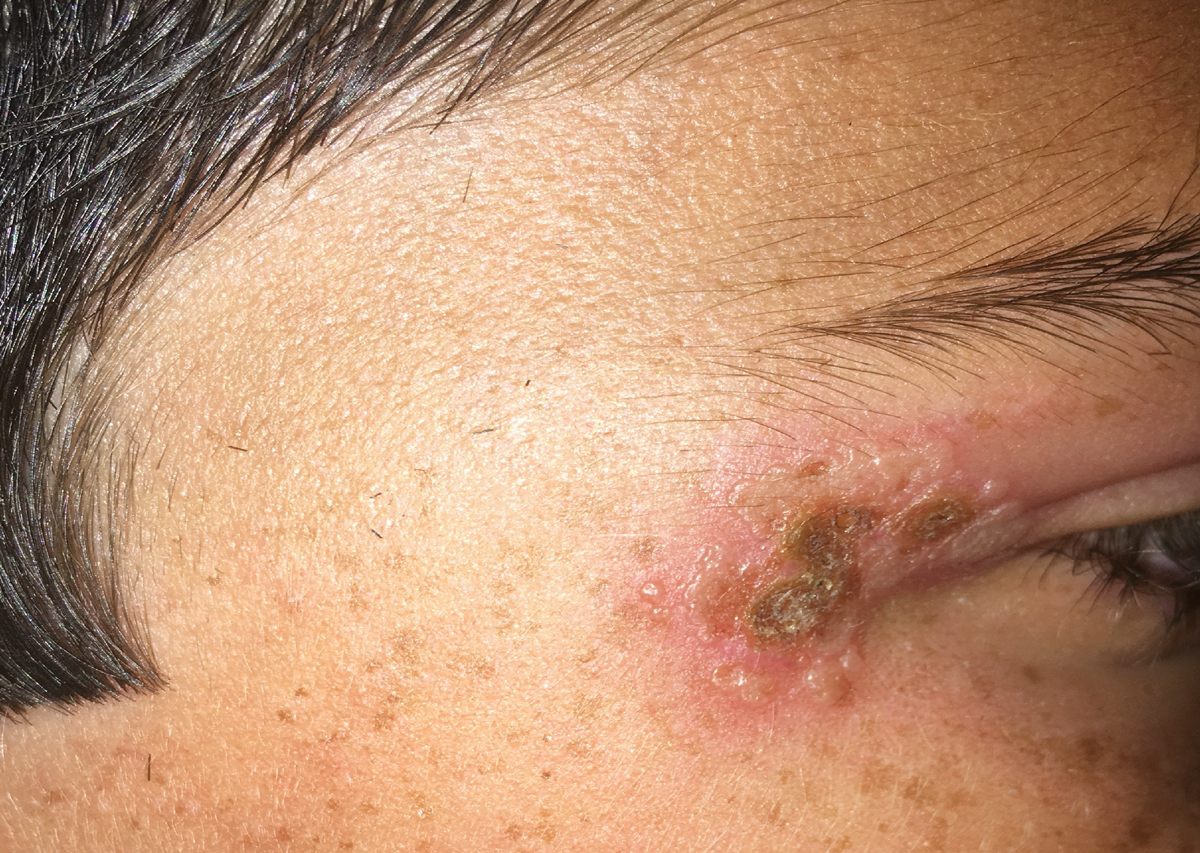 |
|
Primary ocular herpes simplex infection may manifest as blepharitis. Click image to enlarge. |
Considering the approximate equivalency in clinical efficacy, prescriber preference of agent is generally based on dosing frequency, cost and availability (Table 1). Valacyclovir is a prodrug of acyclovir. Due to the improved oral absorption and bioavailability of valacyclovir compared to acyclovir, it has a less onerous dosing strategy and is therefore typically the oral antiviral of choice in treating herpes simplex virus (HSV) ophthalmic infections and herpes zoster ophthalmicus. While both drugs are on-label to treat herpetic viral infections in individuals two years of age and older, the safety of valacyclovir has been evaluated in individuals as young as one month old and is typically considered the pediatric antiviral agent of choice by physician.3,4
Famciclovir is the oral prodrug of penciclovir, which has a different mechanism of antiviral activity than acyclovir but is also effective for the treatment of herpes simplex virus and herpes zoster virus (HZV) with similar efficacy in individuals 18 years and older.1,2,5
Dose and dosing strategy for treatment of HSV-associated anterior segment conditions varies by clinical diagnosis due to the underlying differences in pathogenesis. The dosage for the treatment of varicella zoster virus, the cause of HZV, is at least twice that of the dosage for herpes simplex epithelial keratitis, depending on the agent.6 Herpes simplex keratitis exists along a spectrum of clinical presentation, and dosing strategy and frequency of administration of oral agents may vary based on physician preference.
Table 1. Oral Antiviral Dosing Summary | |||
| VZV | Active HSV: therapeutic dose (dendritic epithelial keratitis) | HSV prophylaxis: stromal keratitis without epithelial ulceration | |
| Famciclovir | 500mg TID x 7-10 days | 250mg BID-TID x 7-10 days | 250mg BID |
| Acyclovir | 800mg 5x/day x 7-10 days | 400mg 3-5x/day x 7-10 days | 400mg BID |
| Valacyclovir | 1g TID x 7-10 days | 500mg BID or 500mg TID x 7-10 days | 500mg QD |
Emerging resistance of strains of HSV-1 to acyclovir should be considered in immunocompromised individuals, especially in those who have had long treatment courses—generally associated with prophylaxis.7 Unfortunately, most acyclovir-resistant HSV isolates are cross-resistant to penciclovir; however, resistant strains may be treated with intravenous Foscarnet (brand name: Foscavir).1
2. My patient has a penicillin allergy, now what?
While 10% of the population reports having a penicillin allergy, prevalence data of true Type 1 immunoglobulin E (IgE)-mediated response to penicillin suggests only about 1% of the population is truly allergic.8 When a patient reports a history of penicillin allergy, a careful history should be taken to understand the medication responsible for the reaction, the kind of reaction, how the reaction was managed and the outcome following the reaction.9,10 Penicillin skin testing should be performed to confirm or rule out penicillin allergy in conjunction with the patient’s allergist or managing physician, which may not be practical in settings requiring acute treatment.9,10 Patients with a true penicillin allergy or IgE-mediated reaction may report symptoms including shortness of breath, wheezing, hives, angioedema and anaphylaxis, which occur immediately or within approximately an hour after exposure to the medication.8-10
 |
|
For patients with a penicillin allergy, cephalosporins could be considered as an alternative treatment option. However, it’s important to gather as much data as possible about the patient and his or her allergy to make an informed choice of an alternative antibiotic to prescribe. (Image courtesy of Randall Thomas, OD.) Click image to enlarge. |
Cephalosporins can be considered in patients with penicillin allergy due to the low potential for cross-reactivity, approximately 2%.10 While the risk of cross-reactivity lessens with later-generation cephalosporins, one commonly used in clinical practice, cephalexin, is a first-generation cephalosporin. The risk of cephalosporin use should be carefully weighed against the potential benefit in these individuals, especially those with history of severe reaction, such as anaphylaxis to penicillin.8,10
In an individual with penicillin allergy, asking them about their successful experience with other antibiotics can help to guide the discussion regarding the risks and benefits of alternative antibiotics including sulfamethoxazole/trimethoprim, azithromycin and doxycycline. Knowing a patient’s medication history is the key to choosing a medication that will give them the least amount of side effects and the best chance for a positive outcome.
It’s important to note that oral fluoroquinolones are rarely prescribed in eye care and have been advised to be reserved for circumstances where no other treatment options exist through the FDA due to their generally unfavorable risk-benefit ratio.11
3. Oral steroids: when is tapering necessary?
Management of acute inflammatory episodes, such as exacerbation of peripheral ulcerative keratitis or thyroid orbitopathy, differ greatly from the management of conditions that require high dose, long-term immune suppression, like giant cell arteritis, due to inherent differences in underlying pathophysiology.12
In general, as the dosage or the duration of systemic steroid therapy increases, so does the risk of systemic adverse effects including adrenal insufficiency and potential for glucocorticoid withdrawal syndrome. Therefore, prescribers often aim to treat with the lowest dosage of systemic steroid for the shortest period of time possible while reaching the required clinical effect.13
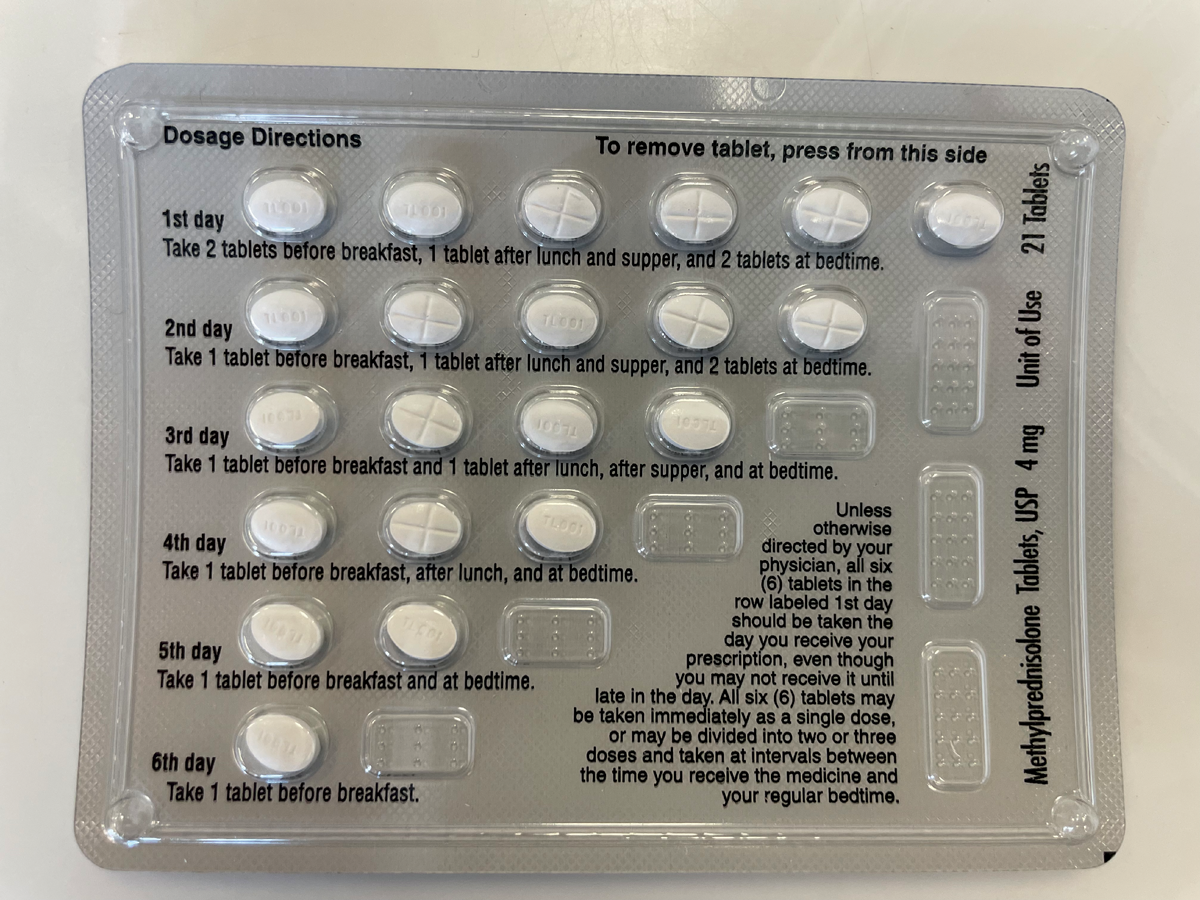 |
|
A methylprednisolone 4mg dose pack with incorporated dosage directions, which can be prescribed to help reduce risk of adrenal insufficiency in patients tapering off steroids. Click image to enlarge. |
Oral corticosteroids like prednisone, prednisolone and methylprednisolone mimic glucocorticoids produced in the adrenal cortex. Endogenous corticosteroid production is equivalent to about 5mg to 7.5mg of prednisolone daily.13 Physiological corticosteroid production is downregulated when exogenous steroids are present due to suppression of the hypothalamic-pituitary-adrenal (HPA) axis. Tapering systemic steroids allows the HPA axis to gradually adjust to the exogenous steroid withdrawal to signal a return of adrenal gland function and increased cortisol production back to physiologic levels while ensuring the underlying disease process remains suppressed.13,14
The rate at which tapering occurs is based on the condition being treated and the duration of treatment. While every clinician has a personal preference when it comes to taper pattern, for patients taking greater than 40mg of prednisolone or an equivalent dosage for more than seven days, a reasonable approach involves decreasing the daily dosage by 5mg to 10mg in weekly increments until 20mg of prednisolone or equivalent is reached, followed by a slow taper of 1mg to 2.5mg weekly until 5mg daily is reached. This slow, sustained taper allows natural cortisol levels to gradually return to a normal range.13,14
Tapering steroids too quickly may cause adrenal insufficiency, resulting in symptoms including headache, fatigue, joint and muscle pain, weight loss, hypoglycemia and fever, which may range from mild to life-threatening.13,14 In addition to a slow taper and minimizing the overall steroid load, to reduce the likelihood of adrenal insufficiency, steroids should be dosed in the morning with as few split doses as possible to mimic circadian adrenocorticotropic hormone release.13
Traditionally, steroid taper is recommended following a treatment with the equivalent of 40mg of prednisolone or greater for more than seven days; however, as adrenal suppression and resulting insufficiency can occur rapidly following the initiation of even low dosages of systemic steroids, a short taper may be included in your indications for use, even for short-term steroid pulses.13,14 A commercially available pre-packaged methylprednisolone dose pack incorporates a pulse followed by a gradual reduction in the steroid over a six-day treatment period, which aids in medication adherence and helps minimize risk of adrenal insufficiency.12
4. Which antibiotics are effective against MRSA?
While methicillin-resistant Staphylococcus aureus (MRSA) infections have been on the decline in the United States from the mid-2000s, the infection and associated multi-drug resistance continue to be responsible for significant morbidity and mortality and are a common cause of periocular soft tissue infection.15-17 Consider the potential for MRSA as a causative organism for individuals who present with periorbital infections and identify as one of the following: patients who work in healthcare environments or were recently hospitalized or incarcerated, athletes, patients with a history of MRSA or who share a living environment with those with a previously diagnosed MRSA infection and those who have non-resolving infections who have been previously treated with a penicillin, cephalosporin or macrolide.15,17
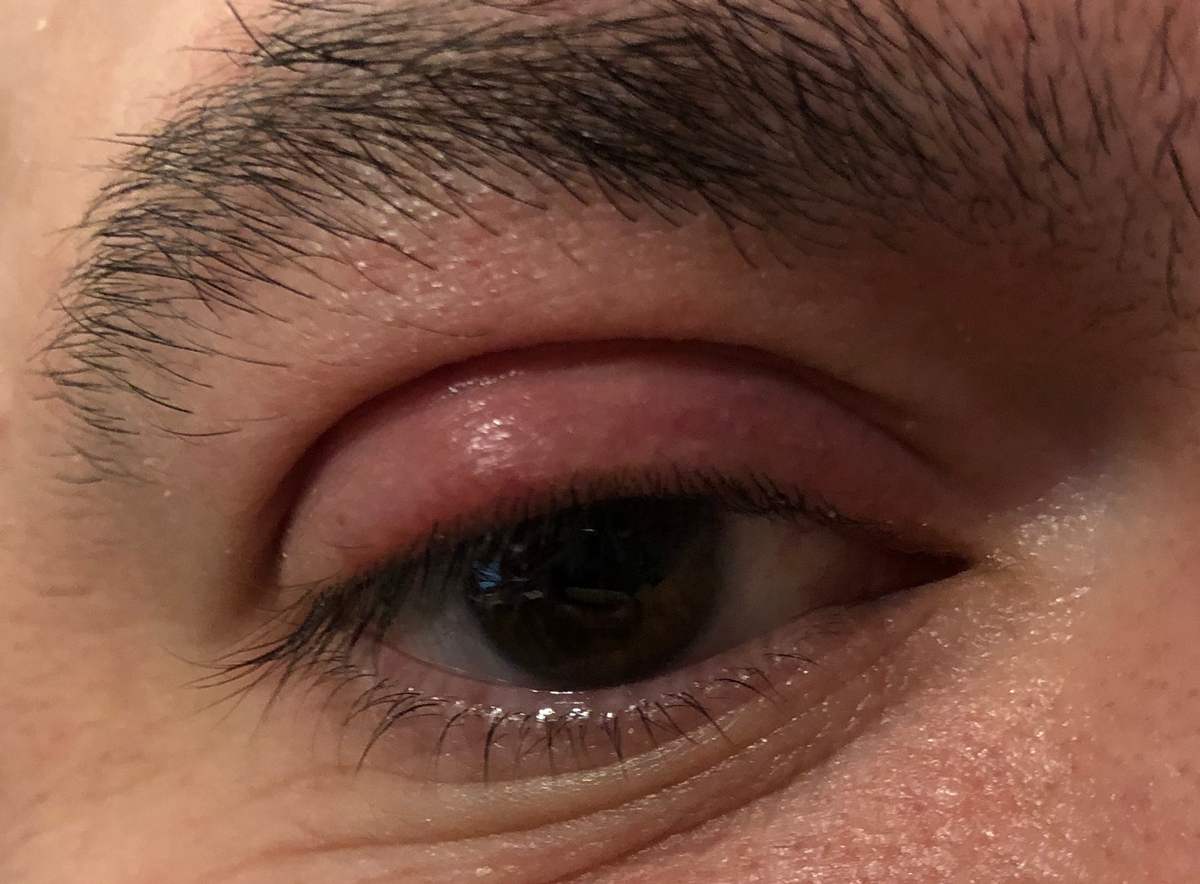 |
|
This patient has an internally-pointed hordeolum of the upper eyelid, a finding commonly associated with MRSA infection. Click image to enlarge. |
Based on known resistance patterns of MRSA in the United States, commonly prescribed oral antibiotic agents in eye care, specifically: penicillins, cephalosporins, and azithromycin, have limited-to-no antimicrobial effect against MRSA.16 Based on individual characteristics and overall individualized risk assessment, while these antibiotics may still be a preferred first-line agent of choice for soft tissue infection, consider the potential for resistance, monitor these patients frequently and adjust the treatment strategy accordingly when limited clinical improvement is observed.
For treatment of suspected MRSA infection, one tablet of sulfamethoxazole and trimethoprim DS containing 800mg of sulfamethoxazole and 160mg of trimethoprim twice daily is the treatment of choice in the absence of sulfonamide antibiotic allergy for most adult patients.16,18 Clindamycin 300mg three times daily or doxycycline 100mg twice daily with food can also be considered when clinically appropriate.16,19,20 A careful risk-benefit evaluation should be performed and discussed with the patient prior to prescription of any oral medication. While all antibiotics alter natural colon flora, clindamycin carries a specific black box warning of Clostridium difficile-associated diarrhea, the development or overgrowth of a severe antibiotic-resistant strain of microbe which may lead to fatal colitis.19
The amount of time to resolution of infections varies, so adjust the follow-up schedule accordingly to optimize the course of treatment based on clinical improvement and resolution of the condition. While some patients may have complete resolution within five days of treatment, others may require 10 or more days.21 Treatment optimization helps limit the overuse of antibiotics.21
Most MRSA strains are susceptible to vancomycin; however, this medication’s use is uncommon in an outpatient setting due to the need for intravenous administration, as well as the concern for judicious use of vancomycin in clinical settings to attempt to delay development of resistance.16
5. Antibiotics in kids—what’s the right dosage and concentration?
Prescribing oral medications for pediatric patients requires an understanding of relevant data regarding the proposed antibiotic, potentially causative pathogen of the infection and individual patient characteristics. While dosage and concentration of medications is often based on patient weight, we know that significant variation in physiological maturation between individuals of the same weight is common, which can result in discrepancies from intended effective dosage resulting in undertreatment, or more likely, increased risk of adverse effects due to overtreatment.22
 |
|
Amoxicillin and clavulanate potassium tablets are commonly prescribed to children and typically dosed based on weight. Click image to enlarge. |
Patients should be encouraged to contact you if they experience adverse effects so the prescribed dosage may be reevaluated.
The most precise prescribing information comes from the package insert of the proposed medication, which is accessible online through the Drugs@FDA: FDA-Approved Drugs webpage.23
Pediatric dosing calculations are always in metric measurements and usually described in milligrams of medication per kilogram of patient weight (mg/kg). A maximum daily dosage will also be included for pediatric dosing, which prescribers should pay careful attention to, especially when prescribing medications for obese children.22
Let’s take a look at an example of a commonly prescribed oral antibiotic in pediatrics: amoxicillin/clavulanic acid. The oral dosage, based on the amoxicillin component for children between three months of age and who weigh 40kg, is 25mg to 45mg/kg/day. The oral suspension is available in four concentrations of the amoxicillin component and two different dosing strategies: 200mg/5mL and 400mg/5mL, dosed every 12 hours, and 125mg/5mL and 250mg/5mL, dosed every eight hours. A 12-hour dosing regimen is typically preferred due to significantly less associated diarrhea.
For a patient with a mild infection, 25mg/kg/day is an appropriate dosage, and for an individual with a more severe infection, 45mg/kg/day of amoxicillin should be considered. For a five-year-old who weighs 20kg with a mild periorbital soft tissue infection, a dosage of 25mg/kg/day every 12 hours calculates to 500mg of amoxicillin daily (with 125mg clavulanic acid), or 250mg twice daily. As each teaspoon (5mL) contains 200mg of amoxicillin, the total daily dosage is 2.5 teaspoons (12.5mL) or 1.25 teaspoons (6.25mL) every 12 hours. When prescribing, keep in mind that oral suspensions need to be shaken well.24
Pediatric prescribing errors are unfortunately common due to the process of multi-step calculations that consider the weight of the individual, dosage of the medication, concentration of the medication and dosing interval. Errors based on weight—where weight may be measured, recorded or communicated incorrectly—are also a frequent cause of prescribing errors.
It may sound obvious, but it’s important to always double-check calculations to limit prescription errors because the risk of miscalculating a dosage is just too high. App-based pediatric dosing calculators, which one can simply download on their smartphone, may also be useful and can minimize mathematical errors.24
The Bottom Line
Nuances in prescribing oral medications take into account a wide variety of ophthalmic condition-, medication- and patient-specific factors. Patient outcomes, as well as their satisfaction with the quality of care they receive, rely on the accuracy of a physician’s treatment and prescribing recommendations. Optimizing medical therapy requires the understanding of the latest available options and careful evaluation and communication of potential risks and benefits to maximize treatment success.
Dr. Steen is an attending optometrist and assistant professor at Nova Southeastern University College of Optometry. She has no financial interests to disclose.
|
1. Biegel JH, Kottilil S. Antiviral Therapy (Non-HIV). In: Goldman, L, Schafer AI, ed. Goldman-Cecil Medicine. 26th ed. Philadelphia: Elsevier, 2020. 2. Tyring SK, Beutner KR, Tucker BA, et al. Antiviral therapy for herpes zoster: randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med. 2000;9(9):863-9. 3. Food and Drug Administration. Highlights of prescribing information: Zovirax. 2005. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018828s030,020089s019,019909s020lbl.pdf. Accessed January 18, 2022. 4. Food and Drug Administration. Highlights of prescribing information: Valtrex. 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020487s014lbl.pdf. Accessed January 18, 2022. 5. Food and Drug Administration. Highlights of prescribing information: Famvir. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020363s037lbl.pdf. Accessed January 18, 2022. 6. White ML, Chodosh J. Herpes simplex virus keratitis: a treatment guideline. Hoskins Center Quality Eye Care and American Academy of Ophthalmology Website. 2014. https://www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline. Accessed January 16, 2022. 7. Rousseau A, Burrel S, Gueudry J, et al. Acyclovir-resistant HSV-1 keratitis: a concerning and emerging clinical challenge. Am J Ophthalmol. 2022;S0002-9394(22)00011-3. 8. Joint Task Force on Practice Parameters; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. 9. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: A practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. 10. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: A Review. JAMA. 2019;321(2):188-199. 11. U.S. Food and Drug Administration. Drug Safety Communication. FDA Drug Safety Communication: FDA updates warning for oral and injectable fluoroquinolone antibiotics due to disabling side effects. 2016. https://www.fda.gov/media/119537/download. Accessed January 16, 2022. 12. Food and Drug Administration. Highlights of prescribing information: Medrol. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/011153s075lbl.pdf. Accessed January 17, 2022. 13. Joseph RM, Hunter AL, Ray DW, et al. Systemic glucocorticoid therapy and adrenal insufficiency in adults: A systematic review. Semin Arthritis Rheum. 2016;46(1):133-41. 14. Prete A, Bancos I. Glucocorticoid induced adrenal insufficiency. BMJ. 2021;374:n1380. 15. Kourtis AP, Hatfield K, Baggs J, et al. Vital Signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible staphylococcus aureus bloodstream infections - United States. MMWR Morb Mortal Wkly Rep. 2019;68(9):214-219. 16. Asbell PA, Sanfilippo CM, Sahm DF, et al. Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2018. JAMA Ophthalmol. 2020;138(5):439-450. 17. Foster CE, Yarotsky E, Mason EO, et al. molecular characterization of staphylococcus aureus isolates from children with periorbital or orbital cellulitis. J Pediatric Infect Dis Soc. 2018;7(3):205-209. 18. Food and Drug Administration. Highlights of prescribing information: Bactrim. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017377s068s073lbl.pdf. Accessed January 18, 2022. 19. Pfizer. Prescribing information: Cleocin. 2021. https://labeling.pfizer.com/showlabeling.aspx?id=625. Accessed January 18, 2022. 20. Food and Drug Administration. Highlights of prescribing information: Vibramycin. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/050006s086,050007s027,050480s052,050533s042lbl.pdf. 21. Llewelyn MJ, Fitzpatrick JM, Darwin E, et al. The antibiotic course has had its day. BMJ. 2017;358;j3418. 22. Conn RL, Kearney O, Tully MP, et al. What causes prescribing errors in children? Scoping review. BMJ Open. 2019;9(8):e028680. 23. Drugs@FDA: FDA-Approved Drugs. 2022. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed January 22, 2022. 24. Food and Drug Administration. Highlights of prescribing information: Augmentin. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050564s053s055,050575s040s042,050597s047s049,050720s026s028,050725s028s030,050726s022s024lbl.pdf. |

