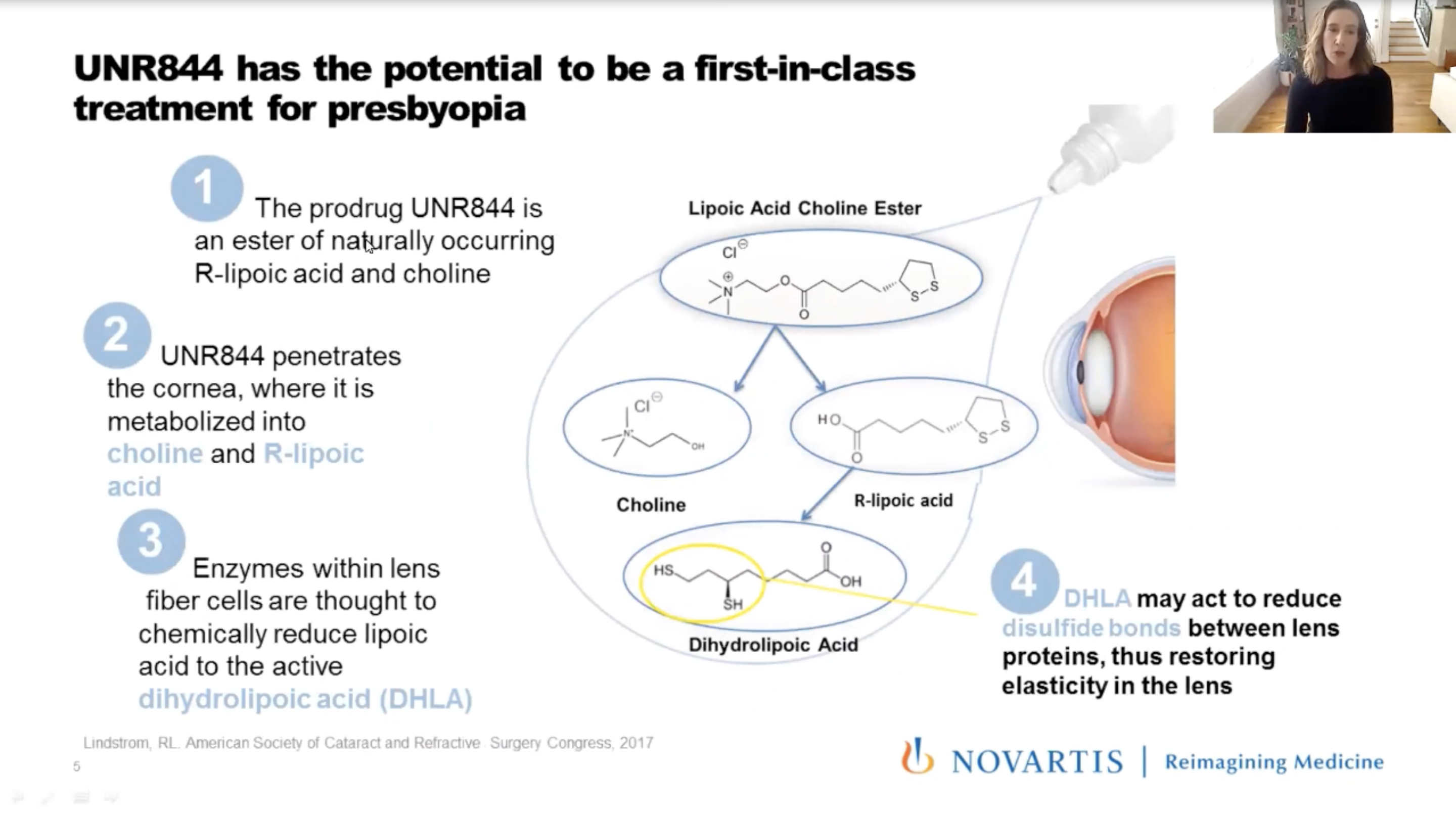
During Academy at Home 2020, Kathryn Richdale, OD, PhD, of the University of Houston College of Optometry, presented the Phase I/II results of the topical eye drop Dioptin (UNR844, Novartis). The eye drop is a lipoic acid-based, topically instilled prodrug that penetrates the cornea. Enzymes metabolized by the crystalline lens help reduce disulfide bonds between proteins and restore elasticity. According to Dr. Richdale, increasing lens protein disulfide content may cause presbyopia through a loss of lens elasticity.
The ophthalmic solution showed a five-letter improvement in distance-corrected near visual acuity (DCVNA) vs. placebo, with treatment benefit observed up to seven months in observational follow-up. The prospective, randomized, double-masked, multicenter Phase IIa study analyzed male and female subjects aged 45 to 65 years old who had DCNVA in each eye and binocularly <70 ETDRS letters (20/40) at 40cm and who needed a minimum add of +1.00D or greater to achieve binocular DCNVA of at least 85 ETDRS letters (20/20.
 |
| During Academy at Home 2020, Dr. Richdale provided attendees information about an eye drop for presbyopia, Dioptin. |
Subjects were randomized 1:1 to receive 1.5% Dioptin (n=40) or placebo drops (n=38) in each eye, twice a day for three months. There was no significant difference in mean change in DCNVA between Dioptin and placebo, (difference of 1.6 letters), so the primary objective was not met. Due to high variability in DCNVA measures in both groups, but to a greater extent in the placebo arm, the researchers performed a post-hoc non-parametric analysis.
The median difference between Dioptin and placebo was four letters closer to the Phase I/II study results, and further clinical development is planned in a Phase IIb dose-finding study. Still, the study noted that future studies should also include mitigation strategies to minimize DCNVA variability. Research is still ongoing to better understand efficacy. Nevertheless, Dr. Richdale states that there is an unmet need for pharmacological treatments in presbyopia.
Richdale K. UNR844 ophthalmic solution for the topical treatment of presbyopia: results of a phase ii randomised controlled trial. Presented at Academy at Home 2020, October 7, 2020. |

