Diabetic retinopathy is one of the most common ocular manifestations of a systemic disease we encounter in primary care optometry. However, it is but one of the debilitating ocular sequelae that affects diabetic patients.
As our scope of practice expands and we increasingly become part of the patients health care team, we must remain aware of the mechanisms behind both the systemic and ocular manifestations of diabetes. As youll see here, these go beyond diabetic retinopathy, although that, too, remains a significant concern.
Epidemiology
The National Health Interview Survey estimates that 6.3% of the U.S. population, or 18.2 million people, had diabetes in 2002.1 Of these, 5.2 million people were undiagnosed. These numbers are expected to rise.
Minority groups suffer disproportionately from type 2 diabetes and its long-term complications compared to whites, possibly due to genetic, lifestyle and socioeconomic factors.2 Indeed, several studies have documented a higher prevalence of insulin resistance in minority groups, particularly blacks, Hispanics and some Native American populations. Some additional risk factors for the development of diabetes include the following: obesity, birth weight of more than 9, a history of gestational diabetes (female patients) and a family history of diabetes.3
Diabetes is now the number one cause of blindness in the 20- to 74-year-old age group in the United States.4 For example, data from two studies found that retinopathy due to type 1 diabetes affects 1 per 300 persons 18 years and older, and 1 per 600 persons with advanced, vision-threatening retinopathy.5 An estimated 5,800 Americans lose their vision each year due to diabetic ocular disease, and diabetic ocular changes currently account for up to 10% of the U.S. blind population.4
Besides ocular disease, diabetic patients compared to non-diabetic patients are 17 times more prone to renal disease and 20 times more prone to gangrene.4 Theyre also five times more prone to myocardial infarction, two times more prone to stroke and eight times more prone to peripheral vascular disease.4
According to the American Diabetes Association, direct and indirect costs of diabetes are approximately $132 billion.
Pathogenesis
Glucose is the preferred energy source for tissues in the human body.4 Gluconeogenesis, a process that occurs in the liver, generates the bodys supplies of glucose. Glucagon, cortisol, epinephrine and insulin all play integral roles in the proper production, regulation and use of glucose.
Insulin, a hormone produced in the pancreas, is the primary factor in the regulation of glucose; it also plays a role in the metabolism of fats and proteins.4 Insulin is secreted by the beta cells in the pancreas. These cells become stimulated when blood glucose levels become elevated (usually after a meal) and secrete insulin. The insulin then binds to glucose and enters the target cell where it starts the oxidation of glucose for ATP production. After the energy needs of the cell have been satisfied, insulin converts any excess glucose into fat where it is stored in the adipose tissues of the body.
Diabetes is a disease of inadequate insulin production and/or use.4 The disease results from an absence of insulin, reduced insulin production, or an inability for receptor sites in target cells around the body to effectively use insulin. Diabetes is usually diagnosed by laboratory testing.4
Diabetes affects all major systems in the body. Optimal management of diabetes requires a multidisciplinary approach that includes the following health professionals: diabetic nurse educator, dietitian, primary care physician, endocrinologist or diabetologist, optometrist or ophthalmologist, retinal specialist, dentist and podiatrist.3,4 (Be sure to document contact information for each health care provider in the patients chart to ensure proper communication.)

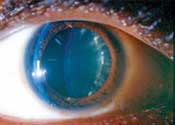
Left: This patient, who has proliferative diabetic retinopathy, also shows subtle iris neovascularization.
Right: A 25-year-old black female with type 1 diabetes and
proliferative diabetic retinopathy exhibits cortical lens changes.
Acute Complications
Hypoglycemia, ketoacidosis and hyperosmolar coma are the most serious complications of diabetes, and are associated with excess mortality.3,6 Indeed, diabetic ketoacidosis (DKA) is the leading cause of morbidity and mortality in children with type 1 diabetes mellitus.7 So, patients who exhibit any of these require prompt medical attention.
- Hypoglycemia. Low blood sugar is the most common acute complication of diabetes, causing morbidity in both type 1 and type 2 diabetic patients, and the condition is sometimes fatal.3,8,9 The usual trigger is insufficient intake of nutrients near peak times of insulin action (usually due to skipping meals), or increased insulin absorption during exercise.3,9
Several common drugs can also enhance the hypoglycemic effects of insulin and oral hypoglycemic agents.3 Alcohol is probably the most common cause of severe hypoglycemia that requires medical attention.3 Other drugs that can lead to hypoglycemia include antibiotics (e.g., doxycycline, tetracycline and chloramphenicol), non-selective beta-blockers and some antidepressants (such as monoamine oxidase inhibitors).3,9
Treatment includes the normalization and stabilization of blood glucose levels, typically with intravenous fluids. It is also important to regulate insulin dosage. - Diabetic ketoacidosis (DKA). This acute complication stems from an insulin deficiency due to infections, a missed insulin dose, or physical or emotional stress.3,4 Specifically, insulin deficiency can trigger glucose production in the liver and reduced glucose uptake, resulting in hyperglycemia; it can also stimulate lipolysis and ketogenesis (conversion of fats to free fatty acids), resulting in ketoacidosis.6
carbon dioxide to normalize the pH.
DKA is characterized by dehydration, abdominal pain secondary to sodium and potassium loss, vomiting, and blood glucose in the 300 to 600mg/dl range.4 The diagnosis is confirmed by blood pH, serum bicarbonate level and serum osmolarity.10
If untreated, DKA will affect almost all physiologic processes in the body, including cardiac activity and the central nervous system. Fluid replacement and stabilization of blood sugar levels are critical elements to preventing fatalities associated with DKA. This usually involves intravenous insulin therapy and potassium supplementation, and careful monitoring of the patients clinical and biochemical status.10
- Hyperosmolar coma. This occurs mostly in type 2 diabetic patients, and continues to be an important cause of morbidity and mortality.11
The mechanisms behind hyperosmolar coma are similar to DKA, however, these patients are usually older diabetics, acutely ill with extreme hyperglycemia (more than 600mg/dl) and minimal ketosis.3 It is characterized by extreme dehydration and neurologic symptoms, which are related directly to the degree of hyperosmolarity.11
Specific signs and symptoms include reduced insulin levels with electrolyte imbalance, severe dehydration, shock, tachycardia, hyperventilation and occasional seizures.3 Classic symptoms of polyuria, fatigue and lethargy, and polydipsia occur more gradually in hypersomolar coma and the neurologic symptoms are often the primary findings.3
Prompt emergency medical attention in patients with hyperosmolar coma is essential. Treatment calls for replacing fluids and electrolytes.
Chronic Complications
Both type 1 and type 2 diabetes affect the micro- and macrovascular circulatory systems.3 Diabetics need to catabolize fats for energy in the absence of glucose, so blood lipid levels often are elevated.4 The increased levels of cholesterol, triglycerides and phospholipids are responsible for the vascular complications.4
- Microvascular disease. Diabetic microvascular disease most commonly affects the capillaries of the retina and kidneys. Pericytes, which provide structural support in the capillary walls, are lost early in the course of the disease due to hyperglycemia.3,13,14 Subsequent damage to the vascular endothelium and basement membrane with capillary dilation can be observed in the retina (via fluorescein angiography), conjunctiva, skin and kidneys of diabetic patients.3 Evidence suggests a correlation between the effectiveness of glycemic control and the degree to which vascular basement membrane damage occurs.3
Abnormalities of the blood platelets can lead to vascular endothelial cell damage and increased vascular permeability and leakage.3 Capillary blood flow can also be influenced by platelet abnormalities.3 Atherosclerotic plaques can sometimes develop in areas of altered capillary blood flow leading to capillary occlusion and hypoxia of surrounding tissues.3
Microangiopathy is considered one of the main causes of mortality and disability in type 1 diabetics.12 In type 1 diabetes, there is a generalized dilation of the microvasculature. This dilation creates elevated capillary pressure and blood flow, and is now considered one of the mechanisms of both renal and retinal microangiopathy.12,14 - Macrovascular disease. The most common macrovascular complications associated with diabetes are peripheral vascular disease, carotid artery disease and coronary artery disease.
Atherosclerosis, the precursor to coronary artery disease and stroke, is more frequently seen in diabetics. This development of significant atherosclerosis is due to microvascular complications and the need for diabetics to catabolize fats and lipids for energy in the absence of glucose.
Accelerated atherosclerosis, and associated microvascular complications predispose patients to coronary artery disease, stroke and peripheral vascular disease. The incidence of myocardial infarction and hypertension is two to three times greater in diabetics compared to the general population.3 Cardiovascular disease is the most common cause of death among diabetic patients.3,14,15 Untreated hypertension significantly increases the risk of coronary disease, congestive heart failure and stroke in all populations. In the diabetic patient, this is further complicated by evidence that chronic hypertension, particularly systolic hypertension, also influences the severity of diabetic retinopathy and nephropathy.3,14
Diabetic peripheral neuropathy, characterized by loss of sensation in the extremities, occurs in up to 70% of diabetics after 20 years duration of illness.3 A rarer form of neuropathy affects blood vessels of the heart and viscera. Visceral neuropathy causes greater disability in patients and may also cause gastroparesis (delayed emptying of the stomach) with symptoms of nausea, vomiting, abdominal cramping and distention; neurogenic bladder, characterized by urinary incontinence and impotence in males; and reduced cardiovascular reflexes.3,14 - Diabetic renal disease. Kidney disease is a complicating factor in more than 40% of deaths among type 1 diabetics, and up to 50% of deaths among diabetic patients younger than age 40.3,12,14,15 Indeed, diabetes accounts for 43% of new cases of end-stage renal disease.1
Screening for protein in the urine (albuminuria) is frequently used to assess kidney function. Even uncomplicated cases of diabetes are characterized by increased levels of kidney filtration.12 After 20 years duration, 33% of type 1 patients and 25% of type 2 patients have some degree of nephropathy.3,12
Proteinuria is common in blacks with type 1 diabetes. Risk factors include male sex, systemic hypertension, poor glycemic control and longer duration of diabetes.16 One study has also shown that overt proteinuria precedes the development of the proliferative form of retinopathy and significantly increases the risk of clinically significant macular edema in blacks patients with type 1 diabetes.17,18 These patients many times are asymptomatic for years despite levels of albuminuria.3
This underscores the importance of routine lab work for all diabetic patients. Improved blood glucose control, anti-hypertensive therapy and dietary protein restriction can help delay the progression of kidney disease.3,12 End stage renal disease is usually treated with dialysis and/or kidney transplantation surgery.
Non-retinal Manifestations
Diabetes can affect nearly every part of the ocular anatomy. The most common non-retinal manifestations of diabetes include:
- Refractive changes. Type 1 and type 2 diabetes can affect the refractive status of the eye. Patients with type 2 diabetes, however, are often unaware of their condition, and routine ocular examination may reveal the initial diagnosis.
Glucose and sorbitol (sugar alcohol of glucose) directly affect the tonicity of the crystalline lens.13 Accumulation of water and byproducts of the sorbitol pathway in the lens cause changes in the serum osmolarity.13,14 As these changes occur, the lens will shrink and swell, causing the refractive changes sometimes seen daily in diabetic patients.13
Generally, when blood sugar increases, so does relative myopia.13 Myopic shifts of up to 4.00D may occur depending on the patients blood glucose level. Conversely, as the blood sugar drops, a relative hyperopic shift may occur.13 During the history portion of the exam, determine the patients blood sugar levels over the preceding two to four weeks. If blood glucose levels have not been stable in this period, you may need to defer dispensing the final refractive correction. - Delayed corneal healing. Delayed healing of the cornea is probably one of the most common anterior segment sequelae of diabetes. A thickened basement membrane decreases the epitheliums ability to adhere to the stroma.13 This may cause minor abrasions of the corneal epithelium to progress into painful and chronic corneal erosions. So, we must use extreme caution when recommending contact lenses for diabetic patients, as they are more susceptible to contact lens-related infections or ulcers.
- Poor pupillary dilation. This, too, is also commonly seen in diabetics with poor glucose control. This results from an autonomic neuropathy involving the sympathetic tissues of the iris.14 The degree to which the pupil dilates poorly has shown to correlate well with the severity of retinopathy and systemic disease.14
When dilating a diabetic patient, a combination of 1% tropicamide and 2.5% phenylephrine usually works the best. Some retinal specialists routinely use 10% phenylephrine to dilate diabetic patients. However, this can have a vasoconstrictive effect if absorbed systemically, which in turn can elevate blood pressure. - Cataracts. Over time, the hyperosmotic forces that cause refractive changes in diabetes can also lead to premature cataract formation. Glycosylation (the process of adding protein to glucose) and accumulation of sorbitol in the lens causes the opacification.13,14
Cataracts occur two to four times more frequently in diabetics compared to the general population.14 One study found a 2.4 times higher relative risk of cortical and posterior cataract in blacks with a history of diabetes mellitus.19
Lens changes initially appear as snow-white opacities in the lens cortex and posterior capsule.13 Eventually, complete opacification can occur involving all layers of the lens. Diabetic cataracts usually cause more severe visual impairment because they frequently occur bilaterally. Unlike senile cataracts, however, normalization of blood glucose levels can cause a complete reversal of the process.13,14
- Glaucoma. Studies vary about whether diabetes is a risk factor for primary open-angle glaucoma.20 Equally questionable is whether all diabetic patients should be screened for glaucoma.
Keep in mind, however, that iris rubeosis frequently occurs in patients with proliferative diabetic retinopathy. These vessels on the iris surface typically are very small and follow an irregular pattern that originates at the pupillary border. They may progress out toward the limbus, where they can occlude the trabecular meshwork of the anterior chamber angle, causing neovascular glaucoma.14 Microvascular disease associated with diabetes may also involve the capillaries of the optic nerve, leaving it more susceptible to glaucomatous damage even from only moderately elevated IOP.14
- Cranial nerve palsies. Demyelinization of the cranial nerves with spontaneous recovery is thought to be the mechanism behind cranial nerve palsies associated with diabetes.14 Most commonly involved are the third cranial nerve, which accounts for up to 40% of cranial nerve palsies associated with diabetes, as well as the fourth and sixth cranial nerves.13,21
Acute, isolated cranial nerve palsies often occur in middle-aged and elderly diabetic patients.13,14,21 Any diabetic patient that presents with the clinical signs and symptoms of multiple nerve palsies should be suspected of having mucormycosis.21 These patients should be admitted to the hospital for immediate medical therapy given the high incidence of fatalities associated with these types of infections.21
Retinal Ischemia
Two separate circulatory microsystems supply blood to the retina: the choroidal circulation, which originates from the ciliary arteries and supplies the oxygen to the rods and cones, and the retinal circulation, which originates from the ophthalmic and the internal carotid arteries.22 Pericytes, which provide structural support for blood vessels of the body, are preferentially lost early in the course of diabetes.
Conversion of glucose to sorbitol by aldose reductase, followed by accumulation of sorbitol, is the possible trigger mechanism for diabetic retinopathy.4 Alterations in the structure of the vascular walls, combined with sticky blood from hyperglycemia, result in reduced perfusion of the capillaries. The result is ischemia and subsequent hypoxia in the retinal tissues.4 The hallmark sign of retinal ischemia and hypoxia due to diabetes is the dropout of retinal capillaries seen on fluorescein angiography.22
Blindness from diabetic retinopathy peaks between ages 30 and 50.4 This risk is also race- and sex-related. Compared to white males, the risk of blindness due to diabetic retinopathy is 3.8% higher in non-white females, and 1.3% higher in non-white males and white females.4 Some 97% of type 1 diabetic patients have some degree of retinopathy after 15 years duration of the disease.4
The development of diabetic retinopathy increases with age and is related to the level of blood glucose control and other factors such as uncontrolled hypertension.4
Diabetic retinopathy typically develops equally between the two eyes. Consider retinopathy with significant asymmetry to be due to a partial blockage of the internal carotid or ophthalmic artery on the side with the lesser-involved eye, and refer these patients accordingly for carotid duplex studies.4
Non-Proliferative Retinopathy
The components that make up non-proliferative diabetic retinopathy are:
- Microaneurysms. The direct result of weakened capillary walls secondary to pericyte loss and endothelial cell damage, these appear clinically as tiny balloon-like protrusions in the retinal capillaries.4 Microaneurysms typically leak fluid into the retina, resulting in retinal edema.4
- Hard exudates. These lipid deposits in the retina represent areas of chronic retinal edema due to leaking microaneurysms.4 Exudates appear as yellowish plaques with well-defined borders and sometimes have a waxy or glistening appearance.23
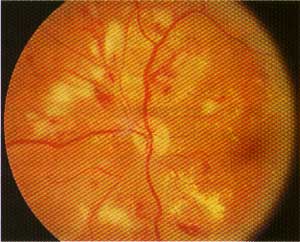
This patient with severe non-proliferative diabetic retinopathy has multiple intraretinal hemorrhages, microaneurysms, hard exudates and multiple cotton-wool spots (superior nasal to the disc).
They typically encircle the area of edema. Their presence around the macula is known as circinate retinopathy. Due to their chronic nature, they indicate a relatively poor prognosis for recovering good vision.4 - Dot-blot and flame-shaped hemorrhages. Dot-blot hemorrhages
represent ruptures in the walls of the capillaries of the deeper retinal layers. They appear as tiny dots, and their shape is due to the column-like anatomy of the retinal layers they reside in, particularly the inner nuclear and outer plexiform layers.24,25
These hemorrhages indicate deep retinal edema.4 An increase in widespread dot-blot hemorrhages indicates retinal ischemia and is a risk factor for progression of diabetic retinopathy and central retinal vein occlusion.4
Flame-shaped hemorrhages are due to abnormalities in the walls of the retinal vessels of the nerve fiber layer. Like dot-blot hemorrhages, flame-shaped hemorrhages also acquire their appearance and name from the striated anatomy of the nerve fiber layer where they are located.24,25 Although frequently seen in diabetic patients, these hemorrhages are most commonly seen in patients with uncontrolled hypertension.4,24 - Cotton-wool spots. Cotton-wool spots are the result of flame-shaped hemorrhages and long-standing hypoxia of the nerve fiber layer.4 They appear as fluffy white lesions that block visualization of the underlying retinal vasculature. They are usually confined to an area in close proximity to the optic nerve (usually within 3 disc diameters).4 The spots themselves are benign, but they may cause small scotomas on threshold visual field testing.4
- Intraretinal microvascular abnormalities (IRMA). Intraretinal microvascular abnormalities, which represent the retinas shunting system, develop in an attempt to drain an area of stasis secondary to severe capillary non-perfusion.4,14 They appear clinically as irregular-patterned, larger-caliber blood vessels within the retina. IRMA are a likely precursor to retinal neovascularization and a relatively ominous sign that the patient may progress to the proliferative form of retinopathy.4 Fluorescein angiography is indicated with IRMA.
- Capillary closure and dropout. Capillary closure is only seen on fluorescein angiography. Look for dark areas in the capillary bed, which is normally lit with fluorescein.4 Capillary closure is one of the earliest signs of retinal ischemia in diabetic retinopathy and a strong argument for obtaining baseline fluorescein studies on diabetic patients.4,22
- Venous beading and tortuosity. Both result from the slowing of venous blood flow due to hyperglycemia.4,14 Tortuosity refers to the irregular pattern of retinal veins. Beading describes the boxcar-like appearance secondary to localized dilation of the retinal veins. They are typically seen with increased retinal ischemia and an indicator to obtain a fluorescein study.
We typically do not refer patients with non-proliferative diabetic retinopathy for laser photocoagulation. Still, we should closely follow any patient who shows a moderate degree of this stage of retinopathy.
Any eye with proliferative diabetic retinopathy has up to a 50% chance of blindness within five years without laser intervention.4 The Diabetic Retinopathy Study (DRS) found that eyes with NPDR and three signs of retinal hypoxia progress to PDR within two years.4 The DRS also illustrated that laser intervention reduced the risk of severe visual loss in eyes with PDR by 50%.4
Every component of PDR has the potential to cause visual impairment.4 The components of PDR include:
- Neovascularization of the optic disc (NVD). This occurs in about 25% of patients with PDR due to hypoxia of the retinal tissue around the optic nerve.4 NVD initially appears as very fine vessels arising from the disc capillaries. It is not restricted by the internal limiting membrane like other retinal vessels and can grow into the vitreous cavity.
All neovascular vessels lack structural support. So they have an increased propensity to leak, resulting in vitreal or pre-retinal hemorrhages. - Neovascularization elsewhere (NVE). This, too, results from retinal tissue hypoxia secondary to capillary non-perfusion. NVE is defined as any vessels that are clearly on the surface of the retina outside of 1 disc diameter from the optic nerve.23 Like NVD, NVE is not restricted by the internal limiting membrane of the retina, and can grow anteriorly into the vitreous cavity. NVE likely sprouts from IRMA in the peripheral retina.4
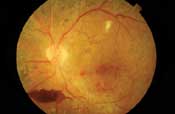
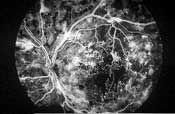
Left: This patient with proliferative diabetic retinopathy has neovascularization of the disc and several tufts of neovascularization elsewhere around the posterior pole. There is a cotton-wool spot with mild venous beading superior to the macula, a pre-retinal hemorrhage secondary to ruptured NVE inferior to the disc and fibrous proliferations along the inferior arcade (seen as yellowish strands).
Right: In the same eye on fluorescein angiography, the dye appears white within the retinal vessels. Microaneurysms and neovascular vessels are easier to appreciate as they are beginning to leak. - Fibrotic proliferations and vitreous scaffolding. Fibrous proliferations result from the growth of NVD and NVE into the vitreous body. These scaffoldings appear as yellowish-white strands or sheets above the retina, and provide the structural support that neovascular vessels inherently lack.23 Because the vitreous becomes more mobile as patients age, these proliferations frequently cause ruptures in the neovascular vessels, leading to vitreous hemorrhages.
After NVD and NVE regress, the vitreous scaffoldings remain.4 Movement of the vitreous creates traction between localized areas of the vitreous base that are still adherent to the retina. Tractional retinal detachments with the potential to cause severe vision loss frequently occur. However, the Diabetic Retinopathy Vitrectomy Study (DRVS) has shown that early vitrectomy in eyes with useful vision and severe proliferative retinopathy releases the traction on the retina and significantly reduces the risk of retinal detachment and vision loss.26 - Vitreous hemorrhage. Hemorrhages above the retina result from ruptured neovascular vessels that emanate from the optic disc and elsewhere in the retina. These hemorrhages are frequently large and obscure the view of the retina. In these cases, B-scan ultrasonography is useful in determining if there is also an associated retinal detachment. Any diabetic patient with a vitreous hemorrhage requires laser treatment to halt the progression and continued bleeding of neovascularization. Hemorrhages typically clear within weeks; however, since laser cannot penetrate blood, non-clearing vitreous hemorrhages should be considered for vitrectomy surgery and intraoperative laser treatment.
Laser photocoagulation destroys retinal tissue.4 This eliminates the need for oxygen to an area of hypoxic retina and halts the vasoproliferative stimulus that creates neovascularization.4 Any eye with signs of PDR warrants an immediate consultation with a retinal specialist, fluorescein angiography and laser treatment.
Macular edema is one of the most common causes of visual loss associated with diabetes. Up to 10% of all diabetic patients will at some point develop macular edema, and up to 4% of these patients will have edema that involves the central fovea.27 It is estimated that there are 75,000 new cases of DME occurring annually.27 Up to 30% of these patients will develop moderate visual loss, defined as a doubling of the visual angle (e.g., 20/40 to 20/80).27
DME can occur at any stage of diabetic retinopathy but does not develop independently from other diabetic retinal changes.14,27 DME is seen in up to 3% of patients with mild NPDR, 40% of patients with moderate to severe NPDR and 71% of patients with PDR.27
DME is classified as either being focal or diffuse.27 Focal DME is caused by microaneurysms that leak fluid into the retina while diffuse macular edema is due to dilated retinal capillaries.27
Capillary hydrostatic forces and tissue oncotic pressure gradients help determine the movement of fluid within the body.27 Disruption of either mechanism can cause a breakdown of the blood-retinal barriers and allow fluid to leak into the retinal tissues. The inner blood-retinal barrier is comprised of the retinal capillary walls, while the retinal pigment epithelium makes up the outer blood retinal barrier. Disruption of capillary hydrostatic forces can come from severe hypertension or any mechanism that causes fluid retention, such as congestive heart failure or renal disease.27 The result is increased capillary hydrostatic pressure that forces fluid from the vascular spaces into the surrounding retina.27
The most common cause of disruption of oncotic pressure gradients results from hypoalbuminemia found in diabetics with renal disease.27 As protein is lost through the urine (proteinuria), a subsequent loss in plasma protein levels results in a decrease of the pressure gradients which causes movement of fluid into the retinal tissues.27
Edema is seen clinically as thickening of the retinal layers.27 We classify the macular edema as either clinically significant (CSME) or not based on its proximity to the central fovea.14 Evidence shows that the risk of moderate visual loss from CSME can be reduced by 50% or more with laser treatment.3 Fluorescein angiography aids in identifying the microaneurysms that cause macular edema and serves as a map for the retinal specialist to apply laser treatment.27
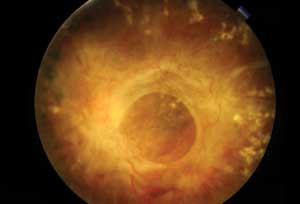 |
| This patient with severe proliferative diabetic retinopathy has dense fibrous proliferation over the macular region. |
Pertinent History
The patient history is one of the most important parts in the examination of the diabetic patient. Pertinent items to document in the history include:
- Type and duration of diabetes. It is important to ascertain the type of diabetes a patient has because type 1 patients typically have retinal manifestations earlier on in life than their type 2 counterparts. Also, type 1 patients are more prone to the acute emergency complications such as diabetic ketoacidosis and hyperosmolar coma. Also, duration of the disease is a significant risk factor for diabetic retinopathy in both types of diabetes.
Sometimes, patients might not be aware of which type of diabetes they have. In this situation, it might help to ask the patient their age at the time of initial diagnosis, as the majority cases of type 1 are diagnosed before age 30.3,14 Remember, just because a patient is on insulin therapy does not always mean that the patient has type 1 since advanced cases of type 2 are characterized by markedly reduced insulin production. - Current therapy regimen. Be sure to document the names and dosages of insulin and hypoglycemic agents that a patient uses. Patients on multiple injections may have difficulty with compliance due to the inconvenient schedule. Poor compliance usually causes frequent visual and refractive fluctuations as discussed earlier. Also, several types of insulin are now available (standard, long, and short-acting). The type of insulin, number of injections taken per day, and number of units taken per injection may indicate how advanced the patients disease is.
- Frequency of blood glucose monitoring and recent blood glucose levels. Some patients may be placed on a sliding scale of insulin. In other words, the number of units of insulin a patient takes depends on blood glucose levels prior to the injections. If a patient on such a scale only checks his sugar once per day or less and is taking multiple injections, you should question how well his or her disease is controlled.
Also, patients whose blood glucose levels fluctuate 100mg/dl or more daily can experience significant refractive shifts. Use your discretion in deciding whether to dispense a final prescription to patients whose blood glucose levels have not been stable for at least two to four weeks prior to their appointment. - Symptoms of other diabetic complications. A patient who is beginning to experience diffuse systemic manifestations of diabetes may be progressing into more advanced stages of the diabetes.
Peripheral neuropathy is a common complication in diabetics. Symptoms of peripheral neuropathy include decreased sensation to hot or cold, pain, numbness, and/or tingling sensations in the arms, legs, hands and feet. Diabetic renal disease creates fluid retention and can facilitate the development or progression of diabetic macular edema. So, you should document whether the patients internist has diagnosed any existing kidney problems, or if the patient is currently in renal failure or on renal dialysis.
You should also confirm whether a patient has hypertension, given that uncontrolled hypertension is a significant risk factor for progressing to diabetic retinopathy. If unsure, it is probably a good idea to take a blood pressure measurement before the exam. Use caution when dilating hypertensive patients with phenylephrine because of the known vasoconstrictive effects with systemic absorption.
Several elements are especially important when examining diabetic patients. These include:
- Tonometry. This is especially important due to the increased risk of optic nerve damage from even moderately elevated IOP.
- Examination of the pupil, iris and anterior chamber angle (with gonioscopy). These should be done before dilation in diabetic patients. Severe enough retinopathy, vitreous hemorrhage or asymmetric retinopathy can cause a relative afferent pupillary defect. Patients can also have a reduced pupillary light reaction after laser treatment. Significant retinal ischemia and subsequent hypoxia can also cause neovascularization of the iris and in the anterior chamber angle, leading to neovascular glaucoma.
- Fundus photography. The Fundus Photograph Reading Center at the University of Wisconsin at Madison, along with the Diabetic Retinopathy Research Study Group, devised a system to allow for accurate and reproducible assessment of the level of retinopathy in a photographed eye.23 In this system, there are seven fields that are photographed. Each field is centered on a particular point of interest in the retina, and several overlap with each other to allow for more accurate grading.23 When photographing a diabetic patient, it is important to follow this protocol to maintain consistency in evaluation.
- Fluorescein angiography. This is the most effective way to observe capillary non-perfusion and identify leaking microaneurysms in diabetic retinopathy. Adverse effects of fluorescein angiography are uncommon, although the patient may experience nausea and vomiting, bruising at the site of the injection, and syncope. It is important to obtain informed consent from the patient before each angiogram. Rarely, patients can experience anaphylactic shock. Therefore, a crash cart should be readily available every time a patient undergoes IVFA.
Dr. Karpecki, director of research for the Moyes Eye Clinic in Kansas City, lectures extensively on new technology, surgical advances and therapeutics, and is the author of Review of Optometrys Research Review column. Mr. Nelson served as an ophthalmic medical research technician at UMDNew Jersey Medical School, Department of Ophthalmology, and was the chief technician on an NIH/NEI-funded study of ocular morbidity and visual impairment of black patients with type 1 diabetes. He is currently a pharmaceutical sales specialist for Bausch & Lomb, and was the inaugural chair of the New Jersey Paraoptometric Section.
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2002. Atlanta: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, 2003.
- Dagogo-Jack S. Ethnic disparities in type 2 diabetes: pathophysiology and implications for prevention and management. J Natl Med Assoc 2003 Sep;95(9):774, 779-89.
- Cavallerano JD. The myriad threats of diabetes mellitus. Rev Optom 1994 Oct 15;131(10):93-110.
- Alexander LJ. Primary Care of the Posterior Segment. 2nd ed. East Norfolk, Conn.: Appleton and Lange, 1994: 232-50.
- Roy MS, Klein R, OColmain BJ, et al. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol 2004 Apr;122(4):546-51.
- Chiasson JL, Aris-Jilwan N, Belanger R, et al. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ 2003 Apr 1;168(7):859-66.
- Dunger DB, Sperling MA, Acerini CL, et al. ESPE/LWPES consensus statement on diabetic ketoacidosis in children and adolescents. Arch Dis Child 2004 Feb;89(2):188-94.
- Radermecker RP, Jandrain B, Paquot N, et al. [Prevention of hypoglycemia in patients with type 1 diabetes] Rev Med Liege 2003 Jun;58(6):361-8.
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003 Jun;26(6):1902-12.
- Yared Z, Chiasson JL. Ketoacidosis and the hyperosmolar hyperglycemic state in adult diabetic patients. Diagnosis and treatment. Minerva Med 2003 Dec;94(6):409-18.
- Reingardiene D. [Hyperglycemic hyperosmolar nonketotic syndrome.] Medicina (Kaunas) 2003;39(7):707-12; quiz 713-4,717.
- Zatz R, Brenner BM. Pathogenesis of diabetic microangiopathy. The hemodynamic view. Am J Med 1986 Mar;80(3):443-53.
- Gurwood A. Endocrine Disease. In: Blaustein B. Ocular Manifestations of Systemic Disease. New York: Churchill Livingstone, 1994:117-48.
- Mahoney B. Diabetes. In: Blaustein B. Ocular Manifestations of Systemic Disease. New York: Churchill Livingstone, 1994:149-63.
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol 1999 Nov;117(11):1487-95.
- Roy MS. Proteinuria in African Americans with type 1 diabetes. J Diabetes Complications 2004 Jan-Feb;18(1):69-77.
- Roy MS. Diabetic retinopathy in African Americans with type 1 diabetes: The New Jersey 725: II. Risk factors. Arch Ophthalmol 2000 Jan;118(1):105-15.
- Roy MS, Klein R. Macular edema and retinal hard exudates in African Americans with type 1 diabetes: the New Jersey 725. Arch Ophthalmol 2001 Feb;119(2):251-9.
- Hennis A, Wu SY, Nemesure B, et al. Risk factors for incident cortical and posterior subcapsular lens opacities in the Barbados Eye Studies. Arch Ophthalmol 2004 Apr;122(4):525-30.
- Ellis JD, Morris AD, MacEwen CJ. Should diabetic patients be screened for glaucoma? DARTS/MEMO Collaboration. Br J Ophthalmol 1999 Mar;83(3):369-72.
- Messner L, Gray L, Modica P. How to Decipher Ocular Motility Dysfunction: A clinical review of third, fourth, and sixth cranial nerve palsies. Rev Optom; 136(9): 94-107.
- Kohner EM: Retinal Ischemia. In: Ryan, et.al. Retina 2nd ed. St. Louis: Mosby-Year Book, 1994:1009-17.
- The Early Treatment of Diabetic Retinopathy Study Research Group: Grading Diabetic Retinopathy from Stereoscopic Fundus Photographs An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology; 98: 786-806.
- Dufek M: Portrait of a Hemorrhage: The Differential Diagnosis of Retinal Bleeding. Rev Optom; 133(7): 82-90.
- Sherman J: Tracking the Source of Retinal Bleeding. Rev Optom; 219(8): 59-66.
- The Diabetic Retinopathy Vitrectomy Study Research Group: Early Vitrectomy for Severe Proliferative Diabetic Retinopathy in Eyes with Useful Vision: Results of a Randomized Clinical Trial DRVS Report Number 3. Ophthalmology; 95: 1307-1320.
- Fareed A: A Review of Diabetic Macular Edema. Dig J Ophthalmol; 1997 Vol 2, No 2, www.djo.harvard.edu/meei/OA/ME/ME.html.

