 |
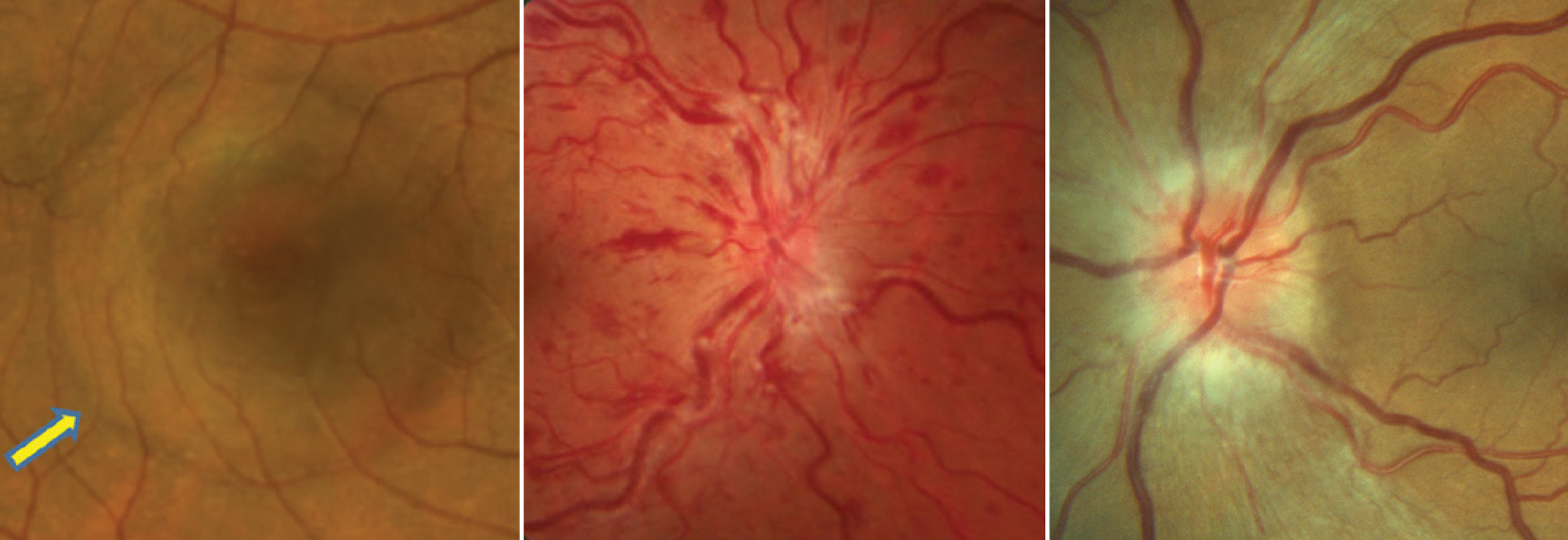
| Study finds elevated risks of (L to R) serous retinal detachment, retinal vein occlusion and ischemic optic neuropathy in patients using ED drugs. Photo: Mohammad Rafieetary, OD, Joseph Sowka, OD, Michael Trottini, OD. Click image to enlarge. |
Several case reports and small epidemiologic studies have quantified the risk of ocular adverse events associated with the use of phosphodiesterase type five inhibitors (PDE5Is). However, results have been conflicting, and epidemiologic data on the risk of serous retinal detachment (SRD) and retinal vascular occlusion (RVO) is not available.
This cohort study with a nested case-control analysis was performed on data from 213,033 men who received PDE5Is at any time over a 15-year period. All four major drugs in this category—sildenafil, tadalafil, vardenafil and avanafil—were included. The case-control analysis included 278 cases of SRD, 628 of RVO and 240 of ION, as well as 4,584 controls. The mean age in both groups was 64.6.
Cohort members were followed up until the first diagnosis of SRD, RVO or ischemic optic neuropathy (ION) or termination of insurance coverage. For each case, four controls were matched by age and time of study entry. Risk for regular users of PDE5Is was compared with that of nonusers, adjusting for potential confounding variables.
Patients with SRD, RVO and ION were more likely to have hypertension, diabetes, coronary artery disease and sleep apnea. The adjusted incidence rate ratio (IRR) for developing any of the three outcomes was 1.85 (15.5 cases per 10,000 person-years). The adjusted IRRs for each individual condition were as follows:
- SRD: 2.58 (3.8 cases per 10,000 person-years)
- RVO: 1.44 (8.5 cases per 10,000 person-years)
- ION: 2.02 (3.2 cases per 10,000 person-years)
“Findings of this cohort study suggest that regular users of PDE5Is might have an increased risk for SRD, RVO and ION,” the study authors concluded in their paper. “Regular users of PDE5Is need to be cognizant of ocular adverse events associated with these drugs and alert their physicians if they experience any visual deficits.”
Etminan M, Sodhi M, Mikelberg FS, et al. Risk of ocular adverse events associated with use of phosphodiesterase 5 inhibitors in men in the US. JAMA Ophthalmol. April 7, 2022. [Epub ahead of print]. |

