 |
|
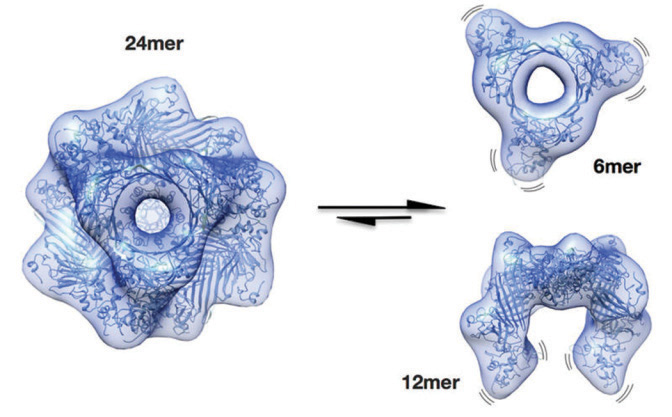
| Researchers learned that protective lens proteins are in a permanently
dissolved state (left). Under stress, they break apart (right) and prevent the other lens proteins from clumping together and forming a cataract. |
|
This breakthrough could be used to create a therapeutic agent to treat cataracts and skip the surgical procedure. In their report, the researchers evaluated the protective effect of two proteins, αA-crystallin and its relative αB-crystallin, which are small, heat shock proteins that help prevent other proteins from clumping together when subjected to significant heat or stress.
Previous researchers have been unable to determine what these protective proteins looked like or how they performed. However, the German scientists made a breakthrough by deciphering the molecular structure of the most important form of the αB-crystallin protein—a molecule comprising 24 subunits.
Under normal conditions (i.e., no stress), this molecule exists in an idle form that contributes little to the prevention of protein aggregation. But when stressed, the protective mechanism of the αB-crystallin molecule is activated. It breaks into smaller units that serve to prevent clumping of other proteins. This finding could lead to the development of a novel medication that would trigger the αB-crystallin activation mechanism, and potentially clear a clouded crystalline lens. Furthermore, because αB-crystallin also plays a role in other tissue cells—including cancer cells—a new agent could be developed to inhibit the protein from interfering with programmed cell death.
Peschek J, Braun N, Rohrberg J, et al. Regulated structural transitions unleash the chaperone activity of αB-crystallin. Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):E3780-9.

