Glaucoma is the second leading cause of blindness worldwide, affecting more than 70 million people.1 In the United States, open-angle glaucoma (OAG) affects roughly 2.2 million people, with an estimated increase of 22% per decade.2
OAG is characterized as primary (POAG) when no cause is identified and secondary otherwise. Most clinicians are on the lookout for the clinical presentations common for POAG, such as optic nerve head (ONH) and retinal nerve fiber layer (RNFL) changes, usually associated with visual field (VF) defects and an open angle on gonioscopy.3 Numerous risk factors associated with POAG also clue clinicians in on a suspected diagnosis, including higher intraocular pressure (IOP), older age, family history, thinner central cornea, lower ocular perfusion pressure, Type 2 diabetes mellitus, myopia, lower systolic and diastolic blood pressure, disc hemorrhage, larger cup-to-disc ratio and higher pattern standard deviation on threshold VF testing.3
Race and ethnicity are important risk factors, as African Americans have a three-fold higher prevalence of OAG relative to Caucasians, and are six times more likely to be blinded by the disease.4-6 The prevalence may be even higher in Afro-Caribbeans.5,6 Moreover, recent studies suggest Asian Americans may have higher rates of OAG comparable with Hispanics, and the latter may have rates comparable with African Americans.7,8
Although prompt detection is crucial to preserving vision, diagnosis isn’t always cut-and-dry, as both POAG and secondary OAG can present with less common forms. Knowing the clinical presentation of these various glaucomas is key to catching unusual suspects early. This article discusses many of the uncommon presentations of glaucoma, as well as the steps necessary to screen patients properly.
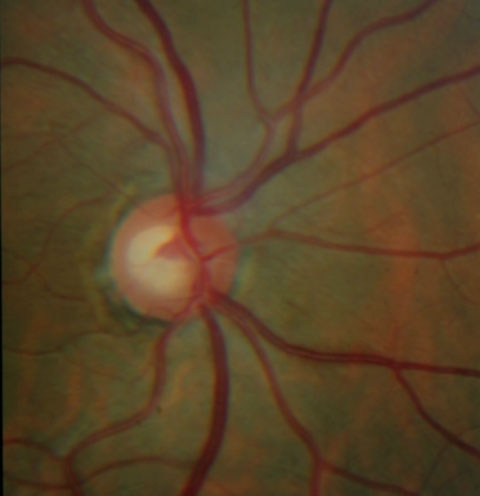 |  |
| These ONH images show an inferior temporal NFL defect with a corresponding rim notch in the left eye in a patient with a glaucomatous optic nerve. | |
Uncommon POAG
Normal tension glaucoma (NTG) is a form of POAG in which IOP is consistently less than 21mm Hg. Although the pathogenesis is poorly understood, research suggests a number of causative factors independent of IOP, including systemic and local vascular dysregulation, hematological abnormalities and structural alterations due to neurovascular dysfunction.9 Researchers believe these factors result in increased sensitivity to a physiologically normal IOP, leading to ONH damage. Systemic conditions that result in ischemic vascular disease, such as diabetes, increase the risk of developing NTG.10 Other contributing factors may include vasospastic events, hypoperfusion, nocturnal hypotension, hypercoagulability and increased blood viscosity and genetics.11 Investigators also found NTG is associated with migraines, especially those with visual auras, and sleep apnea.12NTG is essentially a diagnosis of exclusion after other forms of optic neuropathy have been ruled out. A thorough history helps uncover previous episodes of increased IOP that might explain the optic nerve damage, including inflammation, ocular trauma or past use of systemic, topical, inhaled or nasal steroids.
Signs of NTG differ only slightly from POAG. For one, VF defects appear to be more focal and closer to fixation early in the disease. Ophthalmoscopically, there is a tendency for more localized RNFL defects, and for the presence of disc hemorrhages and peripapillary atrophy (PPA) often associated with the location of the worst cupping. Lastly, central corneal thickness (CCT) tends to be lower in NTG compared with POAG.13
Juvenile open-angle glaucoma (JOAG), a subset of POAG that affects patients between the ages of five and 35, is a rare and usually bilateral condition that affects about one in 50,000 individuals.14 JOAG is an inherited—autosomal dominant—disease in which gene mutations of the trabecular meshwork (TM) proteins lead to increased aqueous outflow resistance. JOAG patients have a male preponderance, a myopic refractive state and frequently present with headaches, in which case neuroimaging is warranted to exclude other potential etiologies.15,16 Secondary glaucomas should also be excluded.
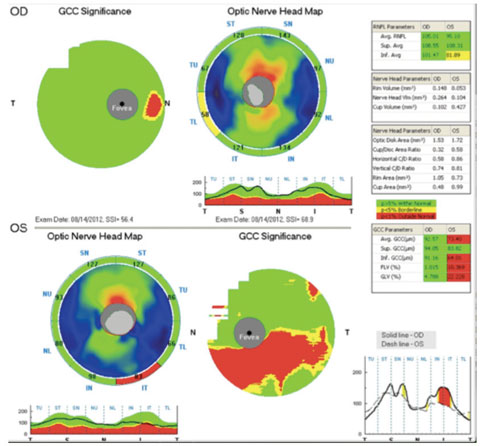 |
| The same patient’s OCT is normal in the right eye, but shows an abnormal inferior temporal RNFL thickness as well as an abnormal inferior GCC thickness in the left eye. |
Secondary Glaucomas
Secondary OAG is a heterogeneous group that includes a wide range of causes and clinical manifestations. Many may initially present as syndromes that require regular screening to monitor their conversion into glaucoma. When confronted with a suspected case of OAG, the differential diagnosis is important, as management may differ.
Pseudoexfoliative glaucoma’s hallmark is the presence of a fine, flaky material on the anterior lens capsule that coalesces into a characteristic bulls-eye or three-ring sign pattern. Peripupillary transillumination defects occur due to the material rubbing on the iris, with deposition of pigment granules on the corneal endothelium, iris surface and TM similar to pigment dispersion syndrome. Gonioscopy may reveal increased patchy TM pigmentation, usually most marked inferiorly, as well as the presence of clear, flaky material.
Because it is the result of a systemic disease, it is a bilateral condition but often presents unilaterally due to significant asymmetry. ON damage and VF loss may occur more rapidly compared with POAG.
Pigmentary glaucoma is typically a bilateral condition occurring when increased IOP is due to iris pigment dispersion and accumulation at the TM. Pigment dispersion syndrome primarily affects young, Caucasian males and patients with myopia. These patients tend to have a more concave approach of the iris, resulting in the pigment epithelium rubbing against the zonular fibers of the lens, which releases iris pigment.
Patients are typically asymptomatic, but episodic rises in IOP secondary to exercise may cause symptoms of colored haloes around lights, blurred vision or subtle ocular pain. Slit lamp examination may reveal a granular brown vertical band along the corneal endothelium known as a Krukenberg’s spindle, pigment deposition on the lens, and spoke-like transillumination defects of the mid-peripheral iris. A dense homogeneous pigmentation of the TM and possibly Schwalbe’s line, leading to a Sampaolesi’s line, is typically seen on gonioscopy.
Traumatic glaucoma results from severe TM dysfunction due to blunt trauma. Clinicians should always suspect traumatic glaucoma and obtain a thorough history whenever they diagnose unilateral glaucoma. Gonioscopy commonly reveals a widening of the ciliary band due to angle recession. Other signs may include irregular and darker pigmentation in the angle, peripheral anterior synechiae or whitening of the scleral spur due to fractured iris processes.
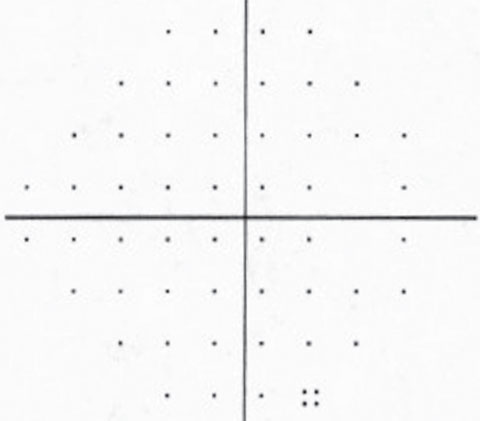 | 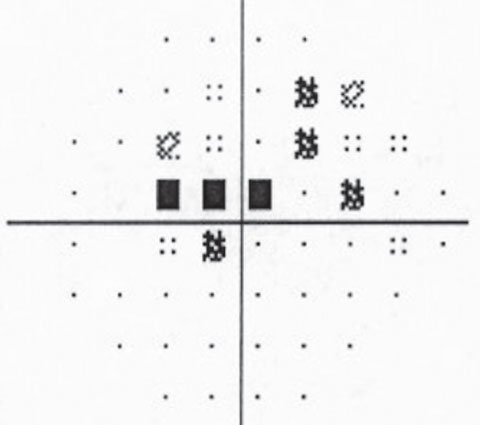 |
| The patient’s visual field is normal in the right eye but shows a superior paracentral defect with the beginning of a superior nasal step in the left eye. | |
Neovascular glaucoma can be an open-angle process in its early stages due to the presence of fine vessels on the TM, leading to its obstruction and decreased aqueous outflow. Angle neovascularization usually occurs due to retinal ischemia, with diabetes and central retinal vein occlusion accounting for most cases.17 Ocular ischemic syndrome should be ruled out when neither of these conditions exist. Due to the pathogenesis of the disease, gonioscopy is crucial in its diagnosis.
Uveitic glaucoma, in its open-angle form, usually occurs when chronic—and sometimes acute—intraocular inflammation causes increased IOP from obstruction of the outflow pathways despite an open anterior chamber angle. Mechanical obstruction by inflammatory cells or dysfunction of the TM is common. In chronic cases, permanent scarring and obliteration of TM beams or Schlemm’s canal may occur. Development of a fibrovascular membrane may also occur due to the release of cytokines.
Biomicroscopic exam reveals cell and flare in the anterior chamber, and gonioscopy may reveal neovascularization of the angle and peripheral anterior synechiae leading to secondary angle-closure glaucoma.
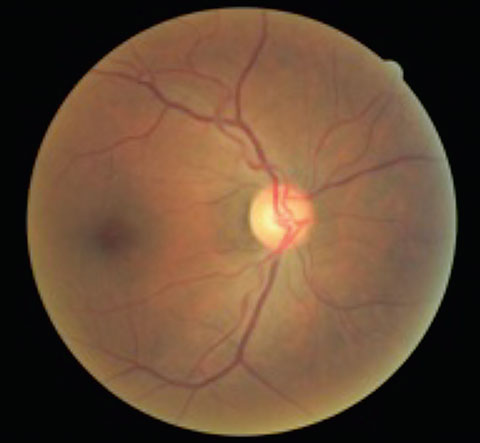 | 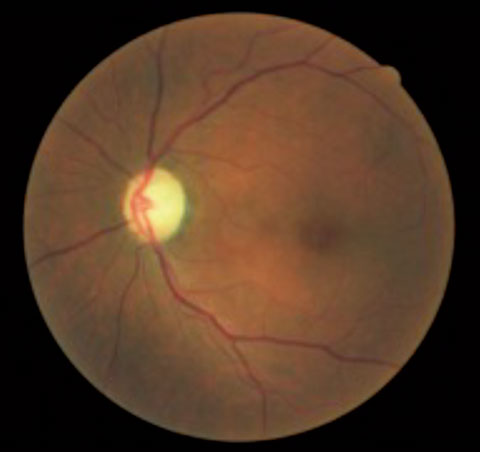 |
| This patient presented with asymmetrical cup-to-disc ratios, as well as ON pallor in the left eye. VFs revealed a bitemporal hemaniopsia, and MRI confirmed the presence of a large pituitary tumor. | |
The Work-Up
With all glaucoma suspects, an extensive history and additional testing are a must. Relevant ocular history includes refractive error, trauma and prior ocular surgery. A history of refractive surgery is associated with a falsely low IOP measurement due to corneal thinning, and cataract surgery may also lower IOP compared with the pre-surgical measurement.18-22 Systemically, clinicians should ask about migraine, vasospasm, diabetes and cardiovascular disease due to their association with POAG in some studies.3 Current medications should be thoroughly reviewed, paying special attention to corticosteroid intake. Race and ethnicity is particularly important, as is the severity and outcome of glaucoma in family members.
In addition to common testing during a comprehensive eye exam, clinicians should obtain gonioscopy, CCT measurement and VF assessment, as well as ONH and RNFL evaluation. Since glaucoma is initially an optic neuropathy that affects the peripheral vision in most cases, VA is usually unaffected until the late stages, although it may be affected in early stages, especially in NTG. A relative afferent pupillary defect in the worse affected eye is expected whenever the disease is unilateral or asymmetric.
Anterior segment evaluation, along with gonioscopy, is essential in the differential diagnosis. Both tests are essential to rule out an angle-closure mechanism, which would require a completely different management. Signs include narrow angles, shallow anterior chamber and peripheral anterior synechiae. Anterior segment evaluation and gonioscopy can also reveal evidence of secondary open-angle mechanisms such as pseudoexfoliation, pigment dispersion, iris and angle neovascularization, angle recession and inflammation.
Fundus examination through a dilated pupil is essential to rule out other abnormalities that may account for optic nerve changes, VF defects or both.3 Additionally, stereoscopic ONH evaluation is paramount in glaucoma diagnosis. Glaucomatous optic nerve changes include vertical elongation of the optic cup, thinning of the inferior or superior neuroretinal rim, excavation of the cup, thinning of the RNFL, notching of the neuroretinal rim and absence of pallor of the neuroretinal rim.3 The ISNT rule indicates that normal nerves show a larger rim width Inferior, Superior, Nasal and Temporal. Glaucomatous optic neuropathy should be suspected when the ISNT rule is not followed, as is the case in 80% of patients with glaucoma.23,24 Other features indicating glaucomatous changes include disc hemorrhages, PPA, nasalization of central vessels and baring of circumlinear vessels.3
The presence of disc hemorrhages does not necessarily confirm a diagnosis of glaucoma, but may be indicative of ongoing focal disc damage and possible progression.25,26 They are observed more frequently in NTG than POAG, and their presence warrants a thorough investigation for glaucoma and clinicians should consider initiating an IOP-lowering therapy.27
PPA—a clinical finding associated with chorioretinal thinning and disruption of the RPE in the area surrounding the disc—is strongly associated with glaucoma and tends to be larger in NTG.28 The presence and extent of PPA, especially the beta zone, has been found to have a strong association with both VF and optic nerve progression.29,30
Research shows structural changes may occur before any evidence of functional change is detected with automated perimetry.31,32 Assessment of structural parameters is therefore important in glaucoma detection, especially when potentially dealing with pre-perimetric glaucoma. In fact, a significant amount of RNFL loss is thought to be required before any visual field defect is detected; thus, quantitative imaging of the RNFL is routinely used as a complement to ophthalmoscopic ONH and RNFL evaluation.3,33
Optical coherence tomography (OCT) can help assess the RNFL thickness at the level of the optic nerve and, more recently, the ganglion cell complex (GCC) thickness at the level of the macula. Both structural assessments provide a statistical analysis that compares the measured thickness to a normative database, as well as a trend analysis over time. However, results from these tests should be interpreted cautiously and within the context of clinical examination, as anatomical RNFL variability may lead to a false positive result compared with the normative database.3,33
Even though GCC distribution tends to be uniform among individuals, concurrent macular conditions may affect the analysis, leading to falsely normal or abnormal results, depending on the condition and software. Additionally, some patients may show VF loss without the presence of corresponding ONH damage, and the sensitivity of functional and structural tests in detecting glaucoma progression may vary depending on the stage of the disease.34-36
Central corneal thickness. Average CCT varies among different races and ethnicities ranging from 534µm to 556µm.3 The World Glaucoma Association cautions against adjusting the IOP, as CCT has been reported to be a risk factor independent of IOP whereby patients with thinner corneas tend to have a higher risk of OAG.37,38 Research suggests that differences in corneal biomechanics may be linked to structural differences of the ocular tissues such as the lamina cribrosa, therefore increasing the susceptibility to glaucomatous optic nerve damage under the same IOP in patients with thinner corneas.39
Visual fields. The gold standard for glaucoma detection and management is automated static threshold perimetry with white-on-white stimulus. Characteristic glaucomatous VF defects are consistent with RNFL damage and include nasal step, arcuate field defect and paracentral depression. Generalized depression may also occur. However, these defects also may be caused by retinal and other optic neuropathies, so the differential diagnosis is important when considering a glaucoma suspect case.
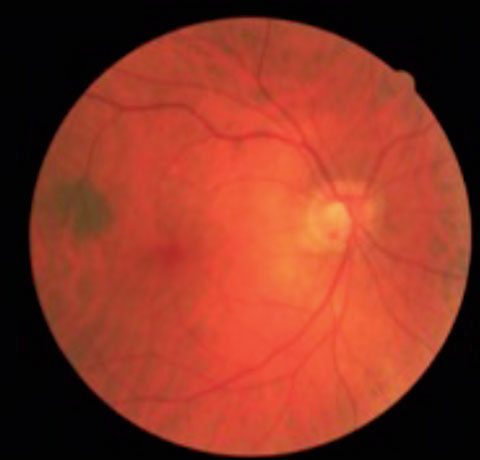 | 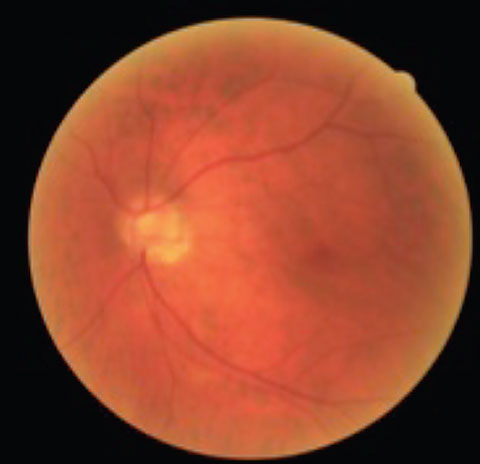 |
| This patient has tilted discs and POAG OU. Note the inferior temporal disc hemorrhage in the right eye. | |
Differential Diagnosis
Optic disc damage and VF defects can be caused by a number of non-glaucomatous disorders—from which glaucomatous damage should be differentiated—such as:
Tilted disc syndrome (TDS) is a congenital anomaly characterized by inferonasal tilting of the optic disc due to incomplete closure of the embryonic fissure. Bitemporal superior VF defects are present in about 20% of patients with TDS and may easily be confused with a chiasmal lesion.40 More rarely, TDS may cause altitudinal or arcuate defects that may be confused with glaucomatous changes.41 The differential diagnosis resides in the fact that TDS is generally non-progressive, and VF defects generally do not respect the horizontal midline.42,43
Compressive optic neuropathy refers to optic nerve damage due to compression by an extrinsic lesion leading to progressive vision loss. Signs that should prompt neuroimaging, especially when facing a potential diagnosis of NTG, include marked asymmetry or unilateral involvement, unexplained vision loss or vision worse than 20/40, VF defects not corresponding to optic nerve head damage, vertically aligned VF defects, presence of other neurological signs, optic nerve pallor in excess of cupping and age younger than 50.44
When faced with a diagnostic dilemma, keep in mind the characteristic features of glaucoma, the differential diagnosis and the clinical presentation of these less common types of glaucoma.
Dr. Makhlouf is an assistant professor with a focus on primary eye care and ocular disease at Nova Southeastern University College of Optometry in Fort Lauderdale, Fla. She teaches the course in ophthalmic lasers and surgical comanagement.
|
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-267. 2. Vajaranant TS, Wu S, Torres M, Varma R. The changing face of primary open-angle glaucoma in the United States: demographic and geographic changes from 2011 to 2050. Am J Ophthalmol. 2012;154:303-14. 3. American Academy of Ophthalmology Preferred Practice Patterns Committee. Preferred Practice Pattern Guidelines. Primary Open-Angle Glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2016. Available at www.aao.org/ppp. Accessed December 6, 2016. 4. Javitt JC, McBean AM, Nicholson GA, et al. Undertreatment of glaucoma among black americans. N Eng J Med. 1991;325(20):1418-22. 5. Friedman DS, Wolfs RC, O’Colmain BJ, et al. Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532-8. 6. Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412-7. 7. Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118:1031-7. 8. Varma R, Ying-Lai M, Francis BA, et al. Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439-48. 9. Mi XS, Yuan TF, So KF. The current research status of normal tension glaucoma. Clin Interv Aging. 2014;9:1563-71. 10. Kim C, Kim TW. Comparison of risk factors for bilateral and unilateral eye involvement in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2009;50(3):1215-20. 11. Stein JD, Challa P. Diagnosis and treatment of normal-tension glaucoma. American Academy of Ophthalmology, EyeNet Magazine. February 2007. 12. Lin PW, Friedman M, Lin HC, et al. Normal tension glaucoma in patients with obstructive sleep apnea/hypopnea syndrome. J Glaucoma. 2011;20(9):553-8. 13. Shetgar AC, Mulimani MB. The central corneal thickness in normal tension glaucoma, primary open angle glaucoma and ocular hypertension. J Clin Diagn Res. 2013;7:1063-7. 14. Turalba AV, Chen TC. Clinical and genetic characteristics of primary juvenile-onset open-angle glaucoma (JOAG). Semin Ophthalmol. 2008;23(1):19-25. 15. Kwun Y, Lee EJ, Han JC, Kee C. Clinical characteristics of juvenile-onset open angle glaucoma. Korean J Ophthalmol. 2016;30(2):127-33. 16. Chak G, Mosaed S, Minckler DS. Diagnosing and managing juvenile open-angle glaucoma. American Academy of Ophthalmology, EyeNet Magazine. March 2014. 17. Shazly TA, Latina MA. Neovascular glaucoma etiology, diagnosis and prognosis. Semin Ophthalmol. 2009;24:113-21. 18. Svedberg H, Chen E, Hamberg-Nystrom H. Changes in corneal thickness and curvature after different excimer laser photorefractive procedures and their impact on intraocular pressure measurements. Graefes Arch Clin Exp Ophthalmol. 2005;243:1218-20. 19. Montes-Mico R, Charman WN. Intraocular pressure after excimer laser myopic refractive surgery. Ophthalmic Physiol Opt. 2001;21:228-35. 20. Rashad KM, Bahnassy AA. Changes in intraocular pressure after laser in situ keratomileusis. J Refract Surg. 2001;17:420-7. 21. Friedman DS, Jampel HD, Lubomski LH, et al. Surgical strategies for coexisting glaucoma and cataract: an evidence-based update. Ophthalmology. 2002;109:1902-13. 22. Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology. 2012;119:1826-31. 23. Harizman N, Oliveira C, Chiang A, et al. The ISNT rule and differentiation of normal from glaucomatous eyes. Arch Ophthalmol. 2006;124:1579-83. 24. Hwang YH, Kim YY. Application of the ISNT rule to neuroretinal rim thickness determined using Cirrus HD Optical Coherence Tomography. J Glaucoma. 2015;24:503-7. 25. Budenz DL, Anderson DR, Feuer WJ, et al. Ocular Hypertension Treatment Study Group. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113:2137-43. 26. Leske MC, Heijl A, Hussein M, et al. Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48-56. 27. Anderson DR. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14(2):86-90. 28. Tezel G, Kass MA, Kolker AE, Wax MB. Comparative optic disc analysis in normal pressure glaucoma, primary open-angle glaucoma, and ocular hypertension. Ophthalmology. 1996;103:2105-13. 29. Martus P, Stroux A, Budde WM, et al. Predictive factors for progressive optic nerve damage invarious types of chronic open-angle glaucoma. Am J Ophthalmol. 2005;139:999-1009. 30. Daugeliene L, Yamamoto T, Kitazawa Y. Risk factors for visual field damage progression in 31. Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109(1):77-83. 32. Quigley HA, Katz J, Derick RJ, et al. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99(1):19-28. 33. Yoo YC, Lee CM, Park JH. Changes in peripapillary retinal nerve fiber layer distribution by axial length. Optom Vis Sci. 2012;89(1):4-11. 34. Chauhan BC, McCormick TA, Nicolela MT, et al. Optic disc and visual field changes in a prospective longitudinal study of patients with glaucoma: comparison of scanning laser tomography with conventional perimetry and optic disc photography. Arch Ophthalmol. 2001;119:1492-9. 35. Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464-70. 36. Leung CK, Cheung CYL, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a study on optical coherence tomography guided progression analysis. Invest Ophthalmol Vis Sci. 2010;51(1):217-22. 37. Dueker DK, Singh K, Lin SC, et al. Corneal thickness measurement in the management of primary open-angle glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2007;114:1779-87. 38. Francis BA, Varma R, Chopra V, et al. Los Angeles Latino Eye Study Group. Intraocular pressure, central corneal thickness, and prevalence of open-angle glaucoma: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2008;146:741-6. 39. Manni G, Oddone F, Parisi V, Tosto A, Centofanti M. Intraocular pressure and central corneal thickness. Prog Brain Res. 2008;173:25-30. 40. Vongphanit J, Mitchell P, Wang JJ. Population prevalence of tilted optic disks and the relationship of this sign to refractive error. Am J Ophthalmol. 2002;133(5):679-85. 41. Brazitikos PD, Safran AB, Simona F, et al. Threshold perimetry in tilted disc syndrome. Arch Ophthalmol. 1990;108(12):1698-700. 42. Dorrell D. The tilted disc. Br J Ophthalmol, 1978;62:16-20. 43. Witmer MT, Margo CE, Drucker M. Tilted optic disks. Surv Ophthalmol. 2010;55(5):403-28. 44. Greenfield DS, Siatkowski RM, Glaser JS, et al. The cupped disc. Who needs neuroimaging? Ophthalmology. 1998;105(10):1866-74. |

