Our understanding of the multifactorial nature of meibomian gland dysfunction (MGD) has evolved significantly since the early 1980s when the term “MGD” was first coined.1 There is now a wealth of in-office interventions practitioners can implement in addition to at-home maintenance therapies.2,3 Despite advancements in MGD treatment, however, these procedures are not fully covered by insurance and can pose a significant cost to the patient. By developing our comprehensive knowledge of MGD therapies, we as practitioners can more effectively identify which procedures will benefit the greatest number of patients, balance improvement in quality of life with cost and build patient loyalty within our practices.
Exploring MGD
The meibomian glands are sebaceous glands located within the tarsal plate of the upper and lower eyelids that produce and secrete meibum, a clear oil in non-diseased states, from orifices anterior to the mucocutaneous junction.4 The muscle force of the orbicularis and Riolan’s muscles contracting during blinking results in dispersal of the meibum from the glands.5 It is this outermost meibum layer of the multilayer tear film that prevents evaporation of the underlying aqueous components.
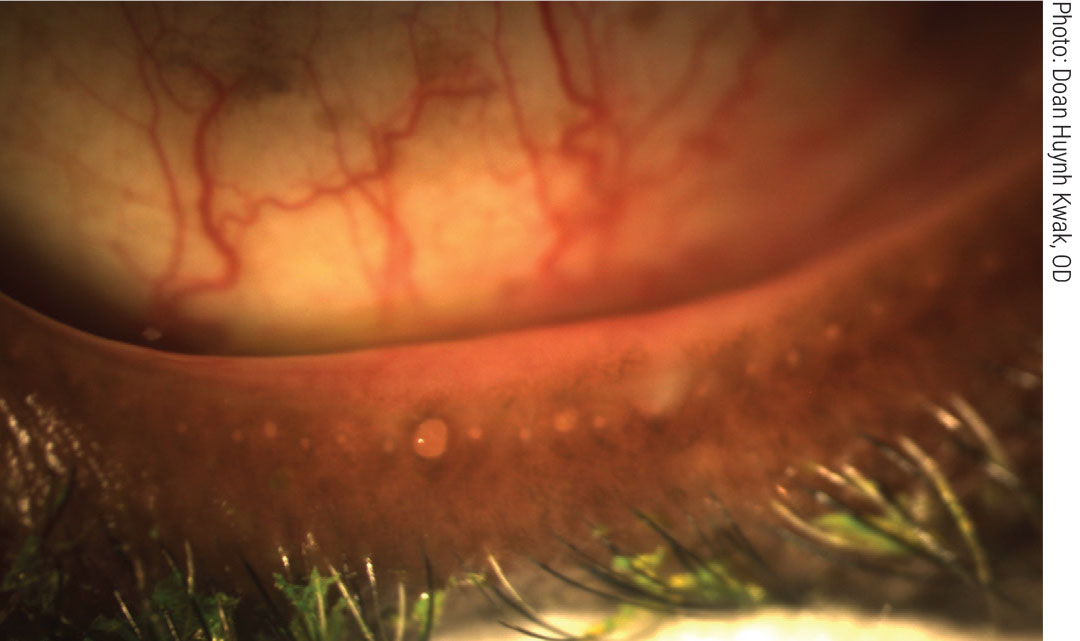 |
|
Obstructed meibomian glands respond to digital pressure by releasing thickened, cloudy meibum. Click image to enlarge. |
Activities that lead to a decreased basal blink rate, such as computer use, can contribute to the development of MGD. Patients with MGD have decreased unsaturated fatty acids and non-polar lipids, resulting in an increased melting point of the secretions. The more severe the meibomian disease, the higher the melting point.6 The melting point of meibum in a non-obstructed gland is 32°C (89.6°F) but can increase up to 45°C (113°F) in severely obstructed glands; this is why therapeutic warming devices should reach temperatures close to 45°C.7
The most common cause of MGD is non-cicatricial obstruction, during which the glands are in their normal anatomic location, but the ducts are obstructed by thickened meibum and keratinized ductal epithelium.1,8 Meibum accumulates in the ducts, causing gland dilatation and distortion. Meibum in the tear film is thus reduced, causing evaporation, increased osmolarity, increased bacterial growth on the lid and inflammation of the ocular surface, followed by atrophic degeneration of the gland in response to increased ductal pressure.4,9 Studies show that obstructive MGD contributes to dry eye in 64% to 87% of cases.5,10
Diagnosing MGD
Accurately diagnosing obstructive MGD requires a combination of patient history, slit lamp exam and use of vital stains. While conducting your slit lamp exam, use your fingers or a cotton tip applicator to perform diagnostic expression of the central to nasal glands of the inferior lid. Press and hold for several seconds, noting the presence or absence of secretions and their quality.
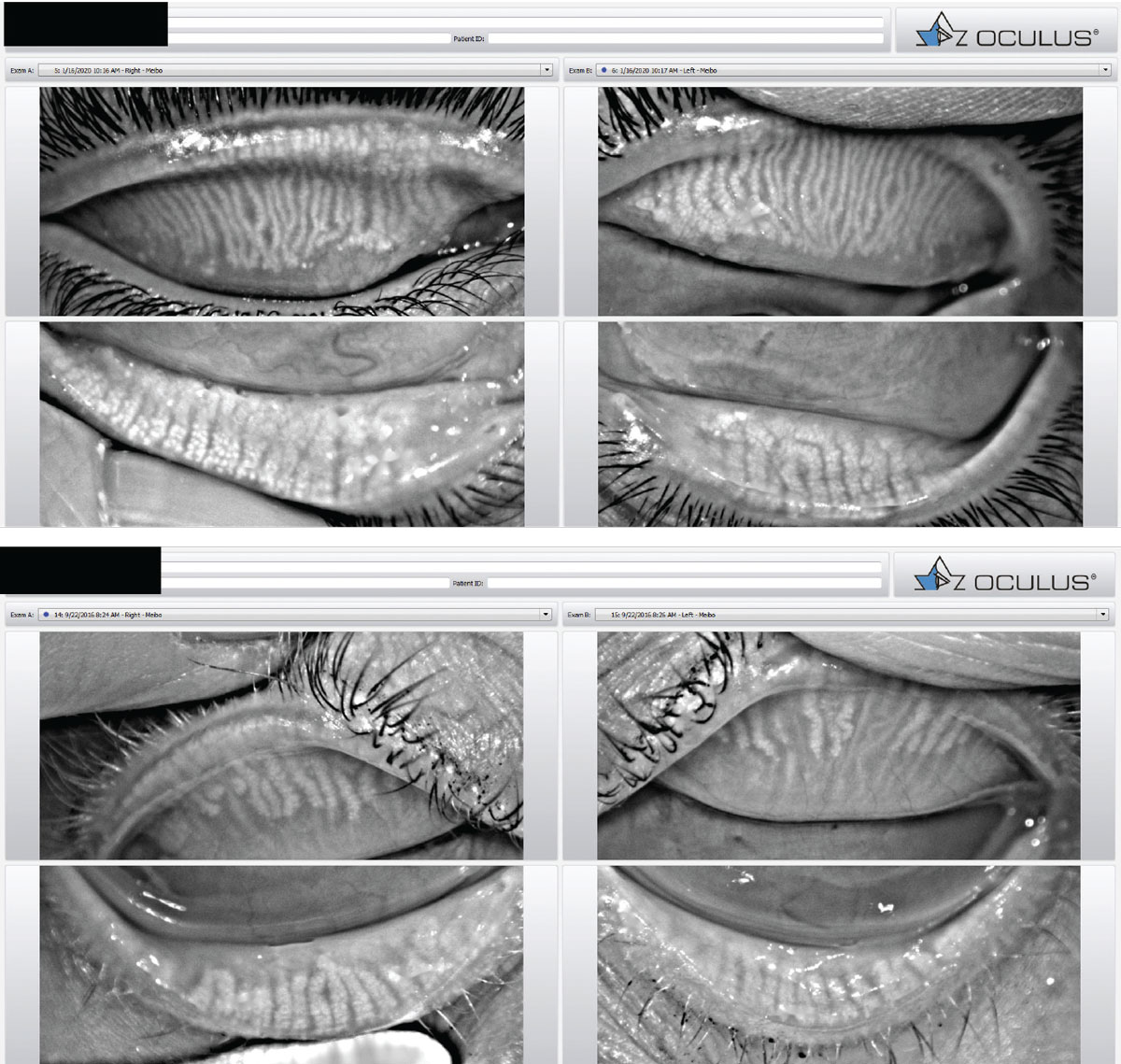 |
|
Meibography images taken with the Oculus Keratograph. Healthy glands are columnar and relatively straight (top). Meibomian gland atrophy associated with MGD (bottom). Click image to enlarge. |
The clinical signs of MGD include meibomian gland dilatation or dropout visible on meiboscopy with lid transillumination, altered gland secretions ranging from cloudy, viscous fluid to a dense, toothpaste-like material, plugging of meibomian orifices, notching and increased vascularity of the lid margin.8,9,11 Infrared meibography can also aid in assessing the distortion and dilatation of glands, areas of atrophy and noninvasive keratographic breakup time of the tear film.12
Treating MGD
For years, the mainstays of MGD treatment have been lipid-based artificial tears, oral tetracyclines, and lid hygiene with warm compresses and lid massage for mild to moderate MGD, and short-term topical steroids for more severe disease.13 Unfortunately, a substantial number of patients remain symptomatic and suffer further blows to their quality of life despite these therapies. Luckily, there are numerous other tools at our disposal to provide patients the ocular relief and improved quality of life they deserve.
What follows is an overview of several interventions for MGD, including a rough estimate of the out-of-pocket price to patients for each (on a scale of 0-4 dollar signs), though practices will naturally vary in what they charge.
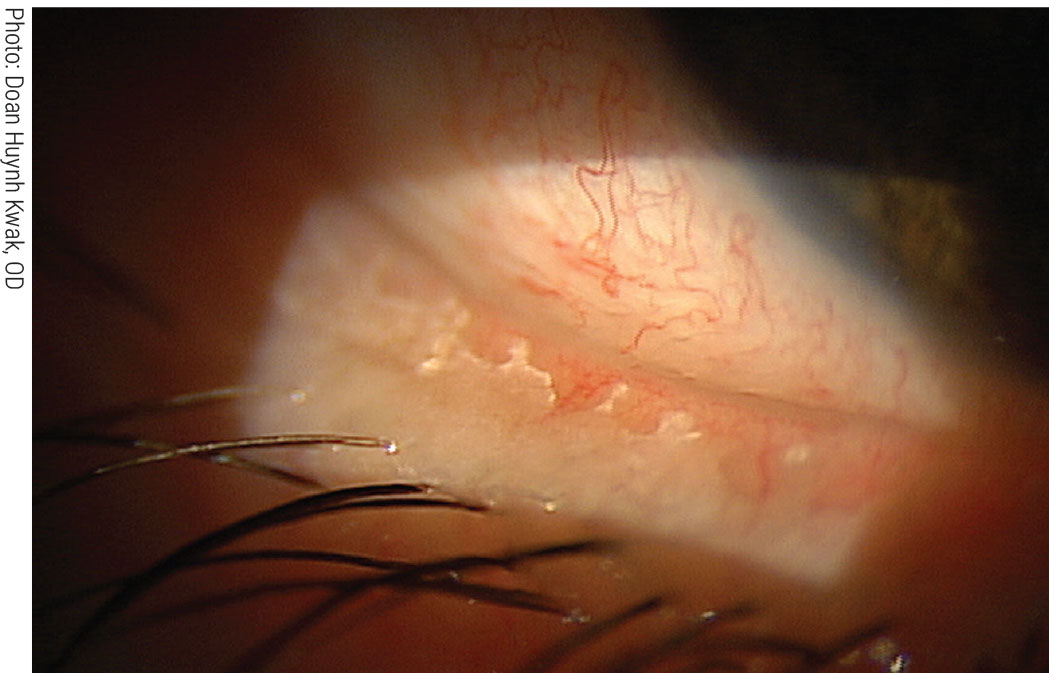 |
|
Removing biofilm from the lid margin via BlephEx reduces the risk of blepharitis and dry eye. Click image to enlarge. |
Therapeutic gland expression (0 to $). Gland expression is key in the diagnosis of MGD, but therapeutic expression has equal value. This is a more comprehensive procedure where the goal is to express all the content within the meibomian glands.
Therapeutic expression can be performed with your fingers or cotton tip applicators, but instruments such as a Mastrota Paddle (Ocusoft) and the Maskin Meibum Expressor (Katena) can ensure a more effective and efficient procedure. For maximum efficacy, the following three-phase approach is recommended: compress, debride, express. Starting with a compress or heating device will significantly liquefy the meibum, making it possible to express the gland content by applying less force to the eyelid.
Next, instill lissamine green in the eye, wait 60 seconds for dispersal and allow the thin mucocutaneous junction (line of Marx) to stain. A stainless-steel golf club spud may be used to apply mild pressure while moving the spud in a lateral motion along this line. No anesthetic is indicated, as only mild pressure is required for debridement. When successfully completed, meibomian orifices should be exposed and there should be little to no oily sheen on the lid margin.14
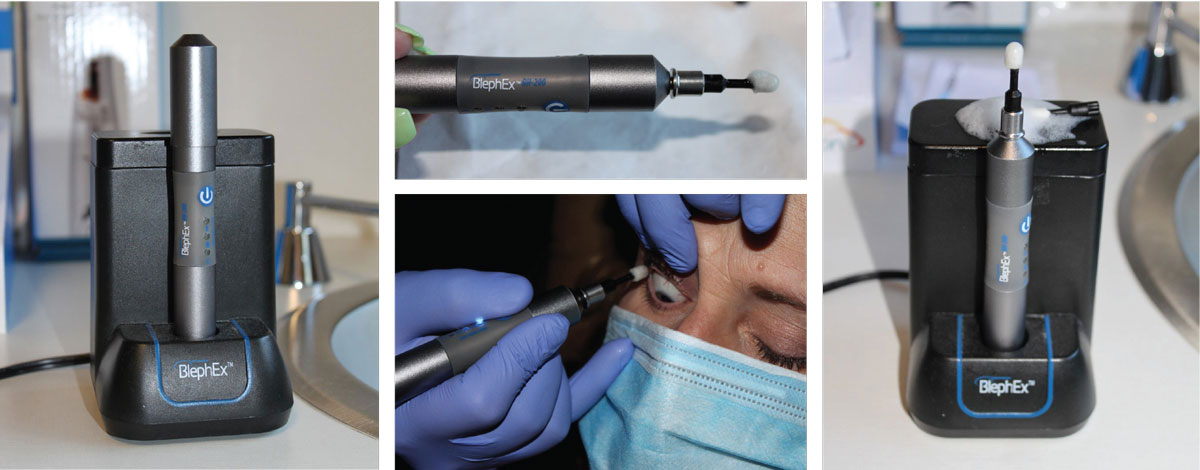 |
|
The BlephEx device for in-office removal of eyelid scurf and bacterial debris, which can cause inflammatory lid disease. The rotating pad buffs away the biofilm and other lid debris to prevent blepharitis. Click image to enlarge. |
To express the inferior glands, pull the lower eyelid down to expose the palpebral conjunctiva and apply an expressor paddle to the nasal palpebral conjunctiva midway between the fornix and lash line while the patient gazes superiorly. Release the lower lid and apply a second expressor instrument to the outer nasal lid. While holding the first device stable, apply force while rocking or moving the external expressor from the base of the glands toward the gland orifices. Several attempts or additional pressure may be necessary if the meibum is turbid. Note areas with no secretions, as this suggests gland atrophy.
Move both expressors temporal on the lid and repeat the process until you reach the outer corner of the eye. For the upper glands, have the patient look inferiorly and gently pull the superior lid away from the globe. Place the expressor paddle underneath the nasal lid midway between the fornix and the lash line and release the lid. Apply the second expressor to the outer nasal lid and repeat the process. Consider topical anesthetic drops and/or a bandage contact lens, depending on practitioner preference and patient cooperation.
Intraductal probing, Katena ($). In 2010, Maskin described a probing technique where stainless steel probes up to 6mm in size are inserted directly into the meibomian gland orifices to unblock meibum or fibrous obstructions within the orifice and duct.15 The procedure uses topical anesthetic in the eye and on the lid while a bandage contact lens protects the cornea.16
The majority of one study’s participants experienced immediate relief in lid tenderness following treatment, with improvements persisting for four weeks, with only 16% of patients requiring additional treatment.17 Subsequent studies additionally showed relative growth of the meibomian glands via infrared meibography. Comparison of pre- and post-treatment images showed reversal of proximal atrophy with lengthening of shortened glands, increased density, partial restoration of glands and the appearance of new glands.18 Growth was consistent for all degrees of gland atrophy.18 While repeated probing is typically necessary, annual retreatment was adequate in these cases.18
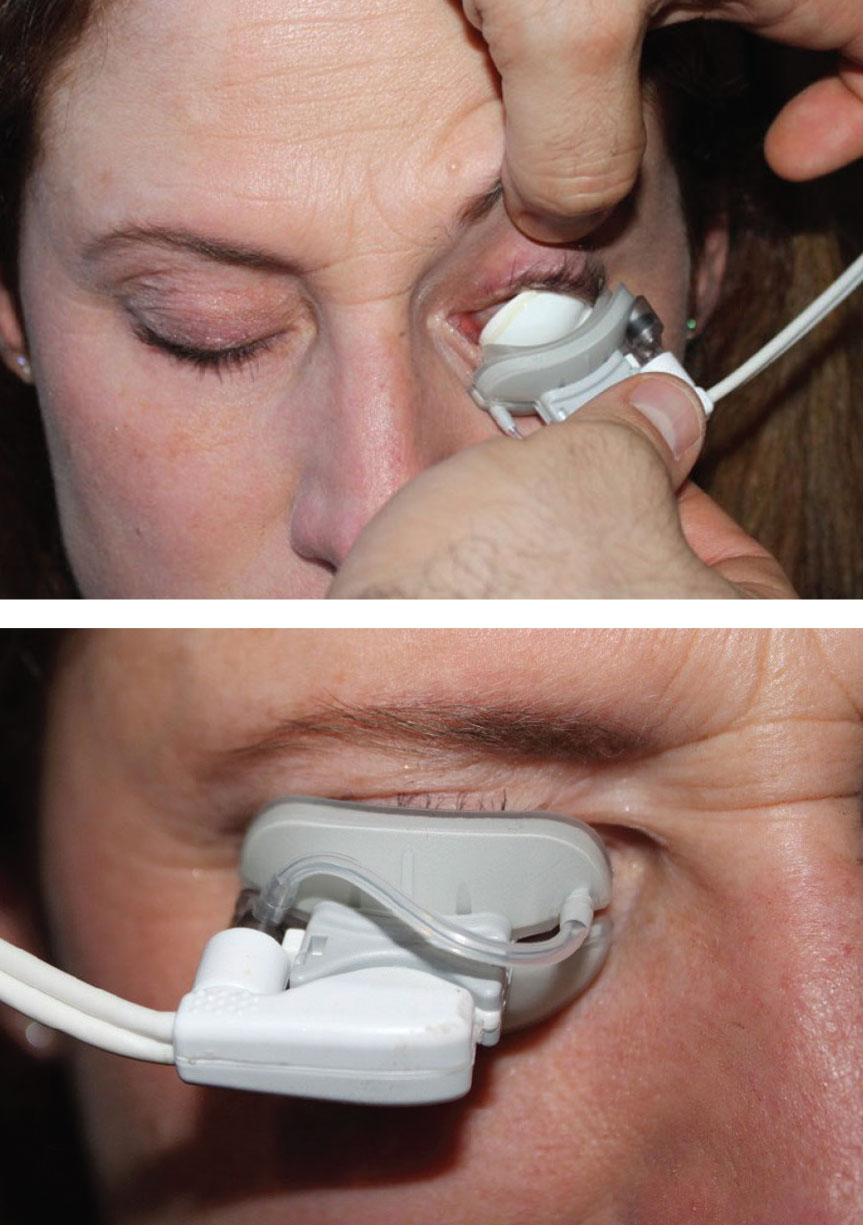 |
|
LipiFlow uses a combination of heat and lid massage to liquefy meibum and clear obstructed meibomian glands. Click image to enlarge. |
LipiFlow, Johnson & Johnson Vision ($$$$). Heat and gland expression are mainstays in the treatment of MGD; however, many of the conventional warming devices apply heat to the outer eyelid surface, limiting the degree and duration of heat that reaches the glands. LipiFlow is an in-office device that takes a different approach by applying heat between 41°C and 43°C to the palpebral conjunctiva while simultaneously applying pulsatile pressure to the outer eyelid surface to express the glands. By directing heat to the palpebral conjunctiva, lower temperatures are sufficient, reducing the risk of thermal injuries.19,20
A study assessed patients who underwent one 12-minute session of LipiFlow and found that at two and four weeks post-treatment, all participants demonstrated clinically significant improvements in meibomian gland secretion and tear breakup time (TBUT).19 At two weeks, 76% reported an improvement in dry eye symptoms, which persisted through the four-week mark.19 Post-treatment improvement in ocular symptoms was sustained for three to nine months in other studies.16,20
LipiFlow has an excellent safety profile, and a single treatment can more effectively improve the symptoms of MGD than a three-month daily course of oral doxycycline without the 40% risk of side effects that doxycycline carries.21,22 The most common findings post-treatment are mild conjunctival injection and mild petechial conjunctival or skin hemorrhages, which tend to resolve within a few weeks of LipiFlow use.
LipiFlow was the first pulsed heat device approved by the FDA and is commonly considered the standard to which others are compared, but it may be cost-prohibitive for some patients, as each session costs $900.
TearCare, Sight Sciences ($$$). TearCare is another in-office warming device but a bit more affordable than LipiFlow, at $600 to $700 per session. Four disposable electrothermal plates adhere to the outer lid and allow the patient to keep their eyes open and blink normally. By allowing the lids to move freely, this device takes advantage of natural meibomian dispersal through blinking. No portion of the device contacts the palpebral conjunctiva or ocular surface.
Connected to the TearCare controller, the plates deliver consistent therapeutic temperatures of 41°C to 45°C for 12 minutes. Following heating, most practitioners perform therapeutic expression to thoroughly evacuate the glands. One study found a statistically significant improvement in signs and symptoms of dry eye at four weeks with TearCare, which continued to six months when the device was combined with therapeutic expression.23
The Olympia study compared the safety and efficacy of TearCare with that of LipiFlow. The initial results indicated that TBUT and meibomian secretion scores saw similar improvements with both treatments, but 90% of patients in the TearCare cohort had a clinically significant decrease in Ocular Surface Disease Index (OSDI) score vs. 79% in the LipiFlow arm. TearCare participants also used 22% fewer lubricant drops post-treatment.24
 |
|
The iLux uses an LED-based heat source to warm the inner and outer lids, then applies gentle pressure to express meibum. Click image to enlarge. |
iLux, Alcon ($$). Relatively new to the market, the iLux is a handheld thermal pulsation device with a disposable patient interface that uses an LED-based heat source to warm the inner and outer lids to the appropriate therapeutic range of 38°C to 42°C. Sensors continuously measure the temperature of the inner and outer lid and turn off the LED if temperatures reach 44°C or 45°C, respectively, to prevent thermal burns.
In a trial comparing iLux and LipiFlow, both treatments significantly improved signs and symptoms of MGD over four weeks with no clinically meaningful difference between the two.25 Treatments typically take less than 10 minutes, and the built-in 15x magnifier on the device allows it to be used anywhere. With results similar to LipiFlow and costing less than it or TearCare, at roughly $300 to $500 per session, this device is an effective, more affordable option.
MiBo Thermoflo, MiBo Medical Group ($$). This device consists of a handheld probe that uses thermoelectric heat to liquefy the meibum within the glands. Ultrasound gel is applied to the probe and used to massage the outer skin of the upper and lower lids for eight to 12 minutes per eye, after which therapeutic expression is performed.26
The Thermoflo II has a dual handpiece, allowing both eyes to be treated simultaneously or separately. Three treatments, each two weeks apart, are recommended initially, and further treatment is determined based on patient response. All portions of the device are reusable, so treatment costs are lower, at $450 for three treatments or $100 to $300 per session.16
There is a relative scarcity of clinical data regarding the MiBo Thermoflo; additional investigation is needed to determine the efficacy and safety profile of this treatment.
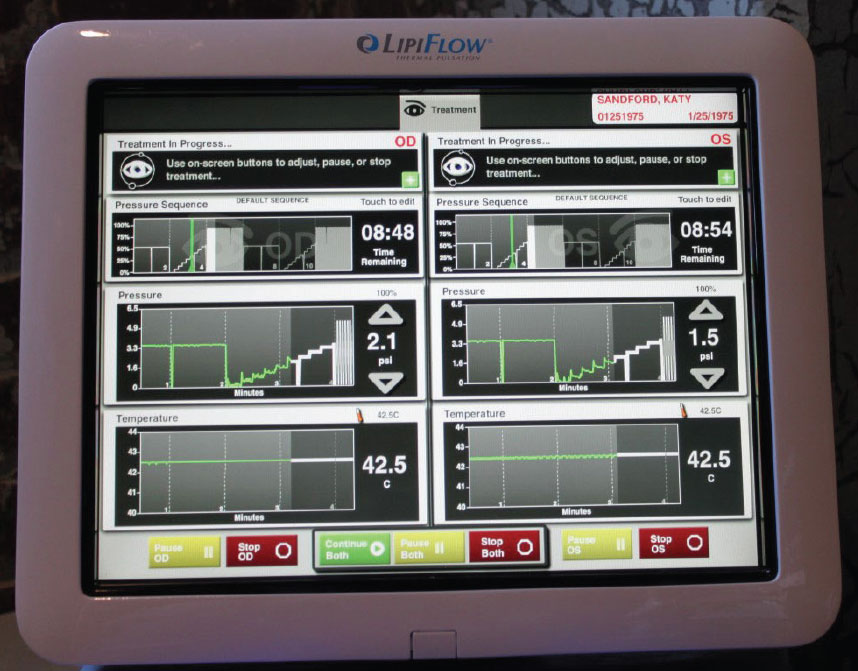 |
|
LipiFlow established the concept of combining heat and pressure for gland expression, and the system offers practitioners useful feedback during the procedure. Click image to enlarge. |
eyeXpress Eye Hydration System, Holbar Medical Products ($$$). Using a goggle-based system, eyeXpress has soft gel inserts that uniformly distribute heat to the upper and lower lids of both eyes simultaneously, all while minimizing the need for pressure on the eye. The goggles are placed over the patient’s eyes for 15 minutes, and therapeutic expression is performed after removal. Typical protocol is four treatments performed three to four weeks apart costing $450 to $750 per session.
BlephEx ($) This is an in-office treatment for exfoliating accumulated debris and biofilm from the lid margin, similar but superior to debridement. The product and the company both use the name ‘BlephEx.’
The dry eye blepharitis syndrome theory asserts that a biofilm comprised of diverse microbes accumulates year after year on the lid margin and is so self-adherent that no home scrub regimen can remove it. As the biofilm thickens over time, bacteria within it proliferate and release inflammatory proteins, leading to blepharitis, hence the correlation between blepharitis incidence and older age. Collarettes, meibomian capping and scurf are all evidence of this layer of biofilm along the lid surface. BlephEx uses a disposable medical-grade micro-sponge that spins on a handheld device to buff away the biofilm in addition to other lid debris, such as Demodex.
BlephEx is often used in conjunction with other procedures but can be used as a standalone treatment. Treatment for all four lids takes seven to eight minutes and is well tolerated by patients. Use of topical anesthetic drops is optional.27 Treatments may be needed as often as three to four times per year; this varies between patients. Each session costs $150. Patients from early studies reported significant improvement in symptoms four weeks post-treatment. All participants exhibited MMP-9 inflammatory markers prior to treatment but tested MMP-9 negative post-BlephEx.28
NuLids, NuSight Medical ($$). While BlephEx achieves a thorough exfoliation of the lid margins in-office, NuLids allows patients to perform lid margin cleanings at home. A drop of gel lubricant or cleaner is placed on the soft silicone tip, which then vibrates when held against the lid. Patients should use NuLids once daily for 15 seconds per lid.
For a list price of around $320, patients can purchase a handheld NuLids from a participating practitioner. Each kit comes with an initial supply of 30 tips. The tips should be disposed of daily, and additional tips can be purchased for $1 apiece.
Intense pulsed light ($$). IPL uses a xenon flashlamp coupled with a filter that emits a wavelength of light within the visible spectrum. Blood cells in abnormal telangectasias absorb this light and coagulate, thrombosing the blood vessel and reducing the release of inflammatory mediators.
This technology has been used for years in dermatology to treat rosacea and acne and has been said to improve MGD and dry eye symptoms in patients treated with IPL for dermatologic disorders.29 Once IPL was more widely implemented for MGD, it was additionally noted that performing meibomian gland expression post-IPL is easier due to the liquefaction of the abnormal meibum and gland dilation.
One commercial application of IPL in eye care is the Optima device from Lumenis, which applies up to 20J/cm2 of energy to the lids and uses a water-cooled tip for patient comfort.
Studies show that even patients with severe MGD demonstrate objective as well as subjective improvement post-treatment. TBUT measurements responded more positively as the number of treatments increased.29,30 Clinical signs improved in 87% of patients, and symptomatology improved in 93%.29,30
Patients can undergo treatments every four to six weeks indefinitely, at a cost of $400 per session. Maintenance IPL treatments are typically required once to twice a year.16 One side effect of IPL is hypopigmentation; therefore, it is important to consider the degree of skin pigmentation a patient has, as lightly pigmented skin is least likely to demonstrate this side effect. Only lower lids are treated to reduce the risk of light absorption by the pigmented structures of the eye, such as the iris.
Low-level light therapy ($$). Like IPL, LLLT also originated in dermatology and has since shown efficacy in managing MGD. Specifically, this therapy can improve TBUT and OSDI scores, especially when combined with IPL.31,32 Unlike IPL, LLLT is athermal, with photoactivation as its presumed mechanism of action, and can safely be applied to the upper lid. Three to four sessions runs about $500.
The Equinox (Marco) is an example of ocular LLLT. The procedure does not involve the use of gel and treats upper and lower lids simultaneously using direct and indirect photobiomodulation during the 15-minute application. The device uses LED lights to deliver focused wavelengths that target the mitochondria, stimulating production of the energy that powers the cell.33
Eye-Light (Topcon), which combines IPL and LLLT, allows for adjusted energy levels based on the patient’s degree of MGD and pigmentation and also does not require gel thanks to a built-in cooling system that maintains an appropriate temperature.31
Warm compresses ($). There are many commercial warm compresses for at-home or in-office use that implement a variety of heating methods. MediBeads (Bruder Healthcare), TheraPearl (Bausch + Lomb) and MGDRx EyeBag (EyeBag Co.) are microwaved to produce dry heat. TranquilEyes XL (EyeEco) uses microwave energy similar to a hot towel compress to produce wet heat. Blephasteam (Thea Pharmaceuticals) and EyeGiene (Eyedetec Medical) use electrical and chemical heat, respectively.
While there is a general lack of agreement on the efficacy of various compresses, there’s definitive consensus that applying 45°C heat for a minimum of five minutes in cases of mild MGD and up to 20 minutes for severe MGD is necessary for adequate therapeutic effect. Patients should not exceed 20 minutes of continuous 45°C heat, as burns can occur.34,35
At-home gland expression (0). For maintenance, patients may perform manual gland expression at home. While looking down, the patient should apply mild force on the nasal upper lid in a downward motion toward the lash line, progressing laterally across the lid to express all glands. The bottom lid should be expressed similarly with the patient looking up. Appropriate supraduction or infraduction during massage prevents direct mechanical force to the cornea, which could lead to corneal deformation. Alternatively, patients may squeeze sections of the upper and lower lids between their index finger and thumb.36
Take-home Message
Currently, in-office MGD therapies are not fully covered by insurance, leaving patients with a large portion of the financial burden. Patient education regarding the benefits of in-office treatment in addition to home therapy is critical in establishing buy-in when it comes to cost and compliance. As practitioners, we must stay abreast of the latest technology and look to clinical data to determine which procedures to implement in our practices for the betterment of our patients’ lives.
Thank you to the TearWell Advance Dry Eye Treatment Center at the FocalPoint Crosstown in Memphis for performing several procedures on me free of charge to help me obtain photos for article use.
Dr. Sanford is an attending optometrist at the Memphis VA Medical Center. She has no financial interests to disclose.
1. International Workshop on Meibomian Gland Dysfunction: report of the Definition and Classification Subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930-7. 2. Hassan A, Balal S, Ahmad S. Meibomian gland dysfunction, dropout and distress: emerging therapies. Eye. 2020;34:1494-6. 3. Schaumberg DA, Nichols JJ, Papas EB, et al. The International Workshop on Meibomian Gland Dysfunction: report of the Subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994-2005. 4. Nichols KK, Foulks GN, Bron AJ, et al. The International Workshop on Meibomian Gland Dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-9. 5. Knop E, Knop N, Millar T, et al. The International Workshop on Meibomian Gland Dysfunction: report of the Subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938-78. 6. Wang MTM, Gokul A, Craig JP. Temperature profiles of patient-applied eyelid warming therapies. Contact Lens Ant Eye. 2015;38(6):430-4. 7. Borchman D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul Surf. 2019;17(2):360-4. 8. Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124(11S):S20-6. 9. Xiao J, Adil MY, Olafsson J, et al. Diagnostic test efficacy of meibomian gland morphology and function. Sci Rep. 2019;9(1):17345. 10. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-83. 11. Tomlinson A, Bron AJ, Korb DR, et al. The International Workshop on Meibomian Gland Dysfunction: report of the Diagnosis Subcommittee. Investig Ophthalmol Vis Sci. 2011;52(4):2006-49. 12. Xiao J, Adil MY, Chen X, et al. Functional and morphological evaluation of the meibomian glands in the assessment of meibomian gland dysfunction subtype and severity. Am J Ophthalmol. 2020;209:160-7. 13. Geerling G, Tauber J, Baudouin C, et al. The International Workshop on Meibomian Gland Dysfunction: report of the Subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050-64. 14. Korb DR, Blackie CA. Debridement-scaling: a new procedure that increases meibomian gland function and reduces dry eye symptoms. Cornea. 2013;32(12):1554-7. 15. Sabeti S, Kheirkhah A, Yin J, et al. Management of meibomian gland dysfunction: a review. Surv Ophthalmol. 2020;65(2):205-17. 16. O’Neil EC, Henderson M, Massaro-Giordano M, et al. Advances in dry eye disease treatment. Curr Opin Ophthalmol. 2019;30(3):166-78. 17. Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010;29(10):1145-52. 18. Maskin SL, Testa WR. Growth of meibomian gland tissue after intraductal meibomian gland probing in patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2018;102(1):59-68. 19. Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396-404. 20. Li B, Fu H, Liu T, et al. Comparison of the therapeutic effect of Meibomian Thermal Pulsation LipiFlow on obstructive and hyposecretory meibomian gland dysfunction patients. Int Ophthalmol. 2020;40(12):3469-79. 21. Hagen KB, Bedi R, Blackie CA, et al. Comparison of a single-dose vectored thermal pulsation procedure with a 3-month course of daily oral doxycycline for moderate to severe meibomian gland dysfunction. Clin Ophthalmol. 2018;12:161-8. 22. Arita R, Fukuoka S. Non-pharmaceutical treatment options for meibomian gland dysfunction. Clin Exp Optom. 2020;103(6):742-55. 23. Badawi D. A novel system, TearCare, for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol. 2018;12:683-94. 24. Sight Sciences presents additional results of OLYMPIA study of signs and symptoms of dry eye disease using TearCare. Eyewire News. eyewire.news/articles/sight-sciences-presents-additional-results-of-olympia-study-of-signs-and-symptoms-of-dry-eye-disease-using-tearcare/. September 9, 2020. Accessed February 16, 2021. 25. Tauber J, Owen J, Bloomenstein M, et al. Comparison of the iLUX and the LipiFlow for the treatment of meibomian gland dysfunction and symptoms: a randomized clinical trial. Clin Ophthalmol. 2020;14:405-18. 26. Kenrick CJ, Alloo SA. The limitation of applying heat to the external lid surface: a case of recalcitrant meibomian gland dysfunction. Case Rep Ophthalmol. 2017;8(1):7-12. 27. Rynerson JM, Perry HD. DEBS—a unification theory for dry eye and blepharitis. Clin Ophthalmol. 2016;10:2455-67. 28. Connor CG, Narayanan S, Miller W. Reduction in inflammatory marker matrix metalloprotenaise-9 following lid debridement with BlephEx. Invest Ophthalmol Vis Sci. 2017;58:498. 29. Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3 year retrospective study. Photomed Laser Surg. 2015;33(1):41-6. 30. Vegunta S, Patel D, Shen JF. Combination therapy of intense pulsed light therapy and meibomian gland expression can improve dry eye symptoms and meibomian gland function in patients with refractory dry eye: a retrospective analysis. Cornea. 2016;35(3):318-22. 31. Stonecipher K, Abell TG, Chotiner B, et al. Combined low level light therapy and intense pulsed light therapy for the treatment of meibomian gland dysfunction. Clin Ophthalmol. 2019;13:993-9. 32. Toyos R, Briscoe D, Toyos M. The effects of red light technology on dry eye disease due to meibomian gland dysfunction. JOJ Ophthalmol. 2017;3(5):555624. 33. Marco Advancing Eyecare. Equinox low level light therapy. marco.com/product/equinox/. Accessed March 2, 2021. 34. Blackie CA, Solomon JD, Greiner JV, et al. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci. 2008;85(8):675-83. 35. Valencia-Nieto L, Novo-Diez A, Blanco-Vázquez M, et al. Therapeutic instruments targeting meibomian gland dysfunction. Ophthalmol Ther. 2020:9(4):797-807. 36. McMonnies CW, Korb DR, Blackie CA. The role of heat in rubbing and massage related corneal deformation. Contact Lens Ant Eye. 2012;35(4):148-54. |

