When sight-threatening conditions strike, they can swiftly cause our patients great detriment. Among these threats, a relatively common occurrence is the corneal ulcer—of which there are several types. Clinicians can evaluate them based on clinical characteristics and patient history, but several of these infections have a similar appearance during various stages of ulceration and can’t be easily distinguished.
| This patient’s corneal ulcer could have any of a number of causes. Culturing the ulcer can provide the materials to identify the etiology and help target treatment. Click image to enlarge. |
The bottom line is, while clinical characteristics and patient history can guide you, they will not always provide the final diagnosis. To reach this, optometrists need a more concrete, objective test. Corneal cultures can help identify the specific type of corneal ulcer you’re dealing with in each case and help you tailor treatment accordingly.
This article provides an overview of how to obtain a corneal culture in your own office as well as how to use it in your management of corneal ulcers.
When to Culture
Consider a variety of factors when deciding if an ulcer needs to be cultured. Small, peripherally-located lesions, or lesions that have no epithelial defect, may be best treated empirically without a culture.1-3
| Collect a small amount of specimen at the ulcer base and at the leading edge of the ulcer. You can use a spatula, spud or swab to achieve this. Here, we are using a spud. Click image to enlarge. |
Culturing may be indicated if the ulcer is large, central, not responding to current treatment or if an atypical infectious organism is suspected.1,4 Because the stakes are higher, consider if the patient is post-surgical, monocular or immunocompromised.2
| We typically use a sterile, cotton-tipped swab for thioglycollate broth and for a quick culture. After collecting this material, it is placed directly into a vial with broth. Click image to enlarge. |
In our clinic, we often refer to the “3-2-1 guideline” when determining when we should culture. This recommends a culture be performed prior to starting treatment if:
(3) the ulcer is 3mm or greater in size at its widest diameter,
(2) there are two or more ulcers,
(1) or the ulcer is within 1mm of the visual axis.
Of course, any guideline has exceptions. For instance, it may be advisable to culture an ulcer that is smaller and peripheral if it has suspicious characteristics. If there is any initial concern for a fungal infection, perform cultures early in the presentation, as it can take up to two weeks for fungus to grow on agar.
It is best to perform cultures before initiating any treatment, as topical medications will decrease the likelihood of obtaining a positive culture result. If you are not comfortable performing the culture in your office, it may be best to refer for this service prior to starting treatment.
Culture Options
A culture on the eye can be performed several ways. The simplest option is called a “quick culture.” This involves collecting a small sample with a sterile swab, often included in the culturing kit. The swab is then placed into the prepared broth and sent directly to the lab, where it will be placed on nutrient plates for identification. A quick culture can be performed on the cornea or on the conjunctiva. There is also an option for transferring the specimen directly to the necessary plates in-office (Table 1). This procedure is described in-depth below.
Table 1. Nutrient Agar Plates | |
| Media | Growth Supported |
| Blood agar | Most bacteria and fungi, except Neisseria, Haemophilus, and Moraxella |
| Chocolate agar | Haemophilus, Moraxella and Neisseria |
| Sabouraud dextrose agar | Fungi |
| MacConkey | Gram negative bacteria only, differentiate lactose positive and negative, which is helpful in identifying Pseudomonas |
| IMA with gentamicin | Fungi |
| Thioglycollate broth | Wide range of bacteria, including anaerobic, and fungi |
| Löwenstein-Jensen medium | Mycobacteria and Nocardia |
| Non-nutrient agar with Escherichia coli | Acanthamoeba |
| Brain heart infusion | Streptococci, meningococci, yeast and fungi |
| Cooked meat broth | Anaerobic and fastidious bacteria |
| These various agars (clockwise from the top: blood, chocolate, IMA with gentamicin and MacConkey) are routinely used to cover a wide variety of organisms. |
Microscopy staining on slides, which can either be interpreted in-office or performed at a laboratory, is another diagnostic test option to consider (Table 2).
In combination, these two tests can differentiate bacteria, fungi, Acanthamoeba and other atypical organisms.
Directly transferring the specimen to the nutrient plates can improve the likelihood of culture growth, but performing a quick culture with the use of transport media allows the lab to prepare its own plates and employ additional agars that you may not have in your office.1 In our clinical experience, performing both types of cultures has increased our overall likelihood for positive growth.
Corneal Scrape and Culturing Procedure
In our clinic, we use the following procedure to perform a corneal culture:
1. Inform the patient about the procedure’s risks and benefits, and obtain proper consent before proceeding.
2. Instill topical anesthetic. Proparacaine is preferred over other anesthetics, as it is less bactericidal.4 A nonpreserved anesthetic should provide the best results.3
3. Align the patient in the slit lamp, as the magnification will be beneficial for the procedure.
4. Using a spatula, spud or swab, scrape the ulcer at its base and at the leading edge of the infiltrate, as the greatest microbial yield will be at these locations. Use enough pressure to indent the cornea slightly. If there is concern for a fungal infection, the scrape needs to be performed deep into the ulcer base to obtain the specimen. If there is significant thinning at the site of the ulcer, it may be best to avoid the base and apply less pressure, so as to decrease the likelihood of a perforation. It is also best to avoid obtaining only purulent material, as it is unlikely to yield a positive result.1,3
| When using a thioglycollate broth, be sure to only hold the handle toward its end—and then to break that end off—so the portion you touch does not contaminate the broth. You may consider wearing sterile gloves for this. |
5. Transfer the specimen to the plate, spreading the material throughout the agar. If you are also preparing slides, place the specimen on the slide first and then the plate.
When preparing plates, avoid breaking the surface of the solid agars. It is convention that a corneal specimen be drawn on the plate in the shape of a “C,” however this is not necessary as long as the plate is clearly labeled as a corneal culture. The specific patterns on the plates become more important if you are also collecting cultures from the lids or conjunctiva.
6. Rescrape the ulcer for each different plate or agar, using a sterilized tool each time. A platinum spatula can be beneficial, as it can be heat-sterilized between specimens, and cools quickly.
| You can also provide a slide for the lab to perform microscopy stains, such as the Gram stain. Here, the specimen is mixed with a sterile solution and covered. Table 2 below indicates which slides can identify which type of organisms. |
If you prefer not to sterilize your tools between scrapes, plate any agars with antibiotic infusion last, such as inhibitory mold agar (IMA) with gentamicin, to prevent the antibiotic in the agar from affecting your results on other plates. If using thioglyocollate broth or a quick culture as part of your testing, use a sterile cotton swab on the ulcer and then place it directly in the broth, breaking the applicator off below the area that you were holding. Do not touch any part of the handle that will be placed into the vial. Wearing sterile gloves can help reduce contamination.
7. Measure the defect after culturing, as there will be greater epithelial disruption following the procedure.
Table 2. Slides for Microscopy Stains | |
| Slides | Organism Identified |
| Gram stain | Bacteria, fungi and Microsporidia |
| Giemsa stain | Fungi, Acanthamoeba and Microsporidia |
| Calcofluor white | Bacteria, fungi, Microsporidia and Acanthamoeba |
| Acid-fast stain | Mycobacterium and Nocardia |
| Grocott-Gömöri mehenamine-silver | Fungi, Acanthamoeba and Microsporidia |
| Periodic acid-Schiff (PAS) | Fungi and Acanthamoeba |
8. Label the plates, vials and slides with information identifying the patient and locations cultured. Fill out the laboratory request form, including information about the site of the ulcer, what plates you are sending for testing and the tests you wish to have performed. You will also want to request sensitivities for medications, including the specific medications that the patient is using or will be starting.
9. Immediately start the patient on empirical-based treatment, if not already begun.
Lab Reports
The lab will communicate to you any positive growth from the cultures. Results may be obtained within a few hours for stains or one to two days for bacterial growth. Slow growing fungal infections can take up to two weeks to show positive growth on plates. Therefore, it may be necessary to begin anti-fungal treatment prior to receiving the culture results if there is a high suspicion for fungal infection.
The lab can also provide sensitivity reports, which will inform you about the effectiveness of various antimicrobials against the isolated organism. These reports are typically sent out at one or two days, seven days or two weeks.3
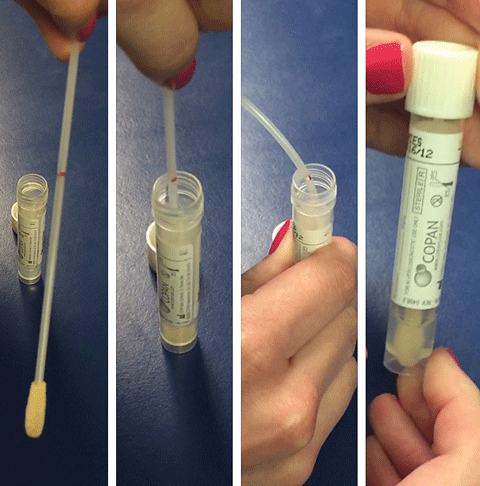 |
| This quick culture kit includes a stale swab along with a prepared broth. Note the red line on the swab’s handle. It’s there to indicate where to hold the handle to prevent contamination. The swab snaps apart at this red line when bent. |
The medication in question may be described as susceptible, intermediate or resistant.
• Susceptible indicates the infectious organism is sensitive to a normal dose of the medication.
• Intermediate means the organism is sensitive to the medication, but only at high dosages.
• Resistant means the organism won’t respond to the antimicrobial.
Antimicrobials classified as susceptible will be the most effective against the infection.
Additional Notes
Culturing may yield a positive result in only 50% to 60% of cases.2 If the patient presents to your office currently on a topical antibiotic, the likelihood of a positive result decreases. It is best to perform a culture before instilling any antibiotic. In some cases, if an ulcer is not responding well to treatment, the provider may discontinue topical medications for a period (usually no longer than 12 to 24 hours) to repeat a corneal culture.1
Proper technique can also help improve test results. If conventional cultures have failed, a corneal specialist may chose to perform a corneal biopsy on the affected tissue. Additionally, a confocal microscope can be a useful, noninvasive tool for ruling out fungi or Acanthamoeba.
Resistance WarningIn the management of bacterial keratitis, the introduction of fluoroquinolones made it more feasible to treat suspected bacterial ulcers empirically, as they could be used to treat a wide variety of common infections. However, the prevalence of antibiotic resistance has been increasing, and even topical fluoroquinolones are affected.1 In fact, approximately 80% of all strains of MRSA are now resistant to fluoroquinolones.2 Also, consider the possibility of fungal, herpetic, atypical bacteria or acanthamoeba—none of which can be treated by a broad-spectrum antibiotic. |
Communication with your lab is key. Plates should be labeled with essential patient identification information. Be sure to specify if you cultured the cornea, conjunctiva or lid. If you want specific agars plated or specific stains performed at the lab, you can make that request. Furthermore, most labs will provide many of the culturing materials to you at no cost, including agars, broths and quick culture kits. If you are interested in culturing in your office, contacting your local lab is the best first step.
For some cases of corneal ulcers, empirical treatment alone is sufficient. However, in cases that do warrant a culture, the procedure must be performed correctly and early. If this is not something you wish to do in-office, learn to recognize which cases should be quickly referred to a provider that will perform the procedure, as an early culture can have a great impact on the outcome for these patients.
Dr. Robinson is from Morehead, KY and graduated from the University of Houston College of Optometry in May 2015. She is the current ocular disease and ocular and refractive surgery resident at the Oklahoma Medical Eye Group and nJoy Vision in Tulsa, OK.
Dr. Ellen graduated from Northeastern State University Oklahoma College of Optometry in 1999 and completed an ocular disease and refractive surgery residency through BVA Advanced Eyecare and TLC Laser Eye Center in Oklahoma City. He serves as the co-coordinator for the Oklahoma Medical Eye Group/nJoy-Tulsa residency in ocular surgery/disease and refractive surgery.
Dr. Hadel is a graduate of the Southern College of Optometry and completed a residency at the VA Medical Center in Kansas City, MO, where he trained in ocular disease and low vision rehabilitation. He practices at the Oklahoma Medical Eye Group where he specializes in the diagnosis and management of ocular disease.
Dr. Lighthizer is the assistant dean for clinical care services, director of continuing education, and chief of both the specialty care clinic and the electrodiagnostics clinic at NSU Oklahoma College of Optometry.
|
1. American Academy of Ophthalmology Cornea/External Disease Panel. Preferred Practice Pattern Guidelines. Bacterial Keratitis. San Francisco, CA: American Academy of Ophthalmology; 2013. Available at: www.aao.org/preferred-practice-pattern/bacterial-keratitis-ppp--2013. 2. Onofrey, B, Skorin L, Holdeman N. Bacterial Keratitis. Ocular Therapeutics Handbook: A Clinical Manual. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2011:165-73. 3. Kanski J, Bowling B. Bacterial Keratitis. Clinical Ophthalmology: A Systematic Approach. 7th ed. Edinburgh: Butterworth-Heinemann/Elsevier, 2011:173-80. 4. Gerstenblith A, Rabinowitz M. Bacterial keratitis and corneal culture procedure. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 6th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;2012:69-73, 435-437. |

