Regardless of who exactly is considered a “baby boomer,” there is no question that the members of this generation have caused a significant demographic impact throughout the last half-century. Now in their mid-40s to mid-60s, it seems that some baby boomers are in denial about their age. Their desire to slow the hands of time has created a skyrocketing demand for aesthetic services and cosmetic enhancement. Economic slowdown aside, this demand to “look as young as you feel” is here to stay.
Cosmetic Considerations
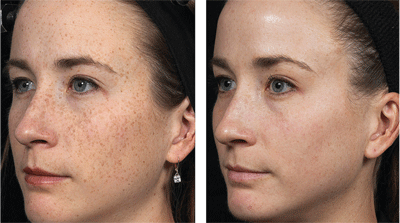
“Before and after” photographs of a patient who underwent a fractional resurfacing treatment for pigment spots. Image courtesy: Solta Medical
As optometrists, we have been participating in the cosmetic industry for several decades. We have addressed patients’ concerns about their appearance by prescribing contact lenses and recommending refractive surgery. Because the first signs of aging usually occur around the eyes, optometrists are in a unique position to assist patients in making aesthetic and cosmetic choices. While Botox Cosmetic (onabotulinumtoxin A, Allergan) and Latisse (bimatoprost ophthalmic solution 0.03%, Allergan) may be available just about anywhere, optometrists who develop solid relationships with reputable cosmetic surgeons and aesthetic consultants can provide great assistance to patients who wish to pursue these services.
The four primary aesthetic side effects of aging are:
• Fine lines and wrinkles.
• Age spots on the skin.
• Sagging skin.
• Increased hair loss or reduced hair growth.
Fortunately, our patients have many choices to address each of these age-related consequences. These options can be divided into injectables, dermal fillers, surgery, lasers and eyelash enhancement.
Injectables
• Botox Cosmetic. Botulinum toxin is a popular, first-line injectable for many patients who want to diminish the appearance of wrinkles caused by genetics, human expression or sun damage. Botulinum toxin type A injections have been the most popular nonsurgical cosmetic procedure during the past five years, with more than 2.4 million total procedures in 2008 alone.1
Botox, a purified protein derived from Clostridium botulinum, has been used for cosmetic enhancement since 2002.2 Botox reduces the release of acetylcholine at the neuromuscular junction. This action tightens the skin and smoothes the lines of expression around the injection spot. While the FDA approved it for the temporary improvement in the appearance of moderate to severe glabellar lines (vertical lines between the eyebrows), Botox is commonly injected in the brow, forehead and/or around the eye to eliminate the appearance of permanent wrinkles and “crow’s feet.” Its effect will last for approximately four months.3
• Dysport. Dysport (abobotulinumtoxinA, Ispen) is a purified neurotoxin type A complex that is produced by fermentation of the bacterium Clostridium botulinum type A-Hall.4 Dysport was approved by the FDA in April 2009 to treat cervical dystonia and the appearance of moderate to severe glabellar lines. However, the drug is being used off-label to reduce the appearance of wrinkles.5
While both Botox Cosmetic and Dysport provide temporary results and are relatively safe, some significant complications can occur.6 The most common complications are bruising or irritation at the site of the injection. In rare cases, patients may also report headache symptoms for up to 48 hours after injection.6
Migration of the medication from the treated area to adjacent tissue can cause unintended consequences, such as droopy eyelids, difficulty swallowing, and perhaps muscle weakness throughout the body. Additionally, patients should not rub the treated areas for 12 hours or lie down for four hours following administration.
Dermal Fillers
Dermal fillers serve as volume correctors and are used to alter the appearance of nasolabial folds, lips, and other facial lines and wrinkles. These products produce immediate patient gratification, with little to no required recovery time. Further, dermal fillers typically last for six months or longer. Some of the most frequently used dermal fillers include collagen, poly-L lactic acid, calcium hydroxylapatite and hyaluronic acid. Below are a few of the most commonly used dermal fillers.
• Collagen. Cosmoplast (Allergan) and Cosmoderm (Allergan) are comprised of human collagen. Cosmoderm is intended to minimize the appearance of unwanted fine lines around the eyes, forehead and glabella, whereas Cosmoplast is indicated for augmentation of the lips.7 The effects of both products last about six months. So, if a patient likes the results, he or she will likely require injections twice a year. Keep in mind that no patient testing is required prior to injection of either product.
ArteFill (Suneva Medical) is the first and only non-reabsorbable dermal filler to receive FDA approval.8 ArteFill provides a permanent support structure for lasting wrinkle correction, which means that its effects are both immediate and long-term.
Artefill is comprised of polymethylmethacrylate (PMMA) microspheres, a material that has been used for years in surgical implants and contact lenses. However, because it is not a natural substance, there is a risk of allergic reaction.8 Generally, complete results are seen within six months after the procedure. Remember, unlike Cosmoplast and Cosmoderm, the results are largely permanent. So, surgical excision is required to reverse any undesired effects.
• Poly-L lactic acid. Sculptra (Sanofi-Aventis), a bio-compatible substance that does not cause damage to surrounding tissues, is the only available poly-L lactic acid. Sculptra is used as a treatment for facial lipoatrophy, a loss of fat beneath the skin that sometimes causes sunken cheeks, indentations and hollow eyes.
Unlike other dermal fillers, Sculptra does not produce immediate results. It works by stimulating collagen production, so results appear gradually over a period of a few months.9 Three to five treatments are usually required, and results can last up to two years or more.
• Calcium hydroxylapatite. Radiesse (BioForm Medical) is made from calcium-based microspheres suspended in a water-based gel.10 Radiesse provides both immediate and extended results because it stimulates production of collagen and encourages tissue regeneration.
• Hyaluronic acid. Hyaluronic acid is a natural substance that is used to maintain skin hydration and volume.11 Common hyaluronic acid fillers include Juvederm (Allergan), Restylane (Medicis Pharmaceuticals) and Hydrelle (Coapt Systems).
Not all dermal fillers are created equal. Each product carries its own list of side effects. The most common complications include asymmetry and/or lumps from poor injection technique.12 Also, injection site pain and infection can occur.
Collagen-based fillers might cause an allergic reaction because they are not synthetically derived. Also, depending on a product’s mechanism of action and composition, it may cause an increased risk of disfigurement, while the effect of a different product may disappear in a matter of months.
A recent survey of members of the British Association of Aesthetic Plastic Surgery indicated that the more long-lasting the dermal filler, the greater the potential for problems.13 According to the survey results, 38.5% of surgeons saw one to three patients who experienced complications with permanent facial fillers during the last year. Further, 23% of surgeons saw one to three patients with severe complications that required surgery. However, the news was considerably better for temporary fillers—81% of surgeons saw no complications when using fillers made from collagen, hyaluronic acid, or other temporary substances.13
You should inquire about what products the cosmetic surgeon or aesthetic center injects and the qualifications of the individuals who are performing the injections. Most adverse events caused by dermal fillers are often the result of an injection performed by an unqualified provider.
Cosmetic Surgery
Patients who wish to eliminate the appearance of significant aging signs, such as sagging skin, often require surgery to achieve a favorable outcome. Eyelid surgery, such as endoscopic brow lift and blepharoplasty, is a very popular cosmetic surgical procedure that you will likely see in clinical practice.
As with other sub-specialists you might work with, be sure to seek the services of a surgeon who limits his or her practice to the performance of plastic surgery. This may be an ophthalmologist with sub-specialty training in ophthalmic plastics and reconstructive surgery, a general plastic surgeon, or even an otolaryngologist.
The key consideration is to work with a surgeon who possesses the knowledge of the necessary procedures and has the ability to perform them. Many surgeons can remove excess skin from the eyelids, but not all can perform state-of-the-art cosmetic eyelid surgery that will not only provide the appearance of younger skin, but also achieve a good functional outcome.
Cosmetic eyelid surgery, unlike medically necessary eyelid surgery, does not require pre-authorization by a third-party payer. Therefore, neither preoperative visual field testing nor preoperative photographs are required (although photographs typically are taken to provide patients with a “before and after” comparison).
Remember, the decision for cosmetic surgery is one made between the patient and the physician. By working with a surgeon who is experienced in eyelid surgery, you will likely see fewer postoperative complications, such as exposure keratopathy.
Laser Treatments
Laser treatment can be used to soften wrinkles, treat age spots on the skin and remove unwanted hair. While laser treatments tend to be expensive, their effects tend to be more permanent and precise than injectable enhancements alone.
When used in conjunction with injectables and cosmetic surgery, laser treatments can be a great way to rejuvenate skin. There are a variety of aesthetic lasers in use and their mechanisms of action vary. Here are a few examples:
• IPL (intense pulse light)
• Nd:YAG
• Alexandrite
• Pulsed dye
• Erbium
• Diode
• CO2 and fractional CO2
• Radio-frequency (not a laser)
Clearly, the potential for ocular injury exists when using any type of laser. Additionally, there is a slight danger of electrical shock or fire as well as infection due to the inhalation of smoke or tissue splattering.
Each laser system has its own adverse effect and complication profile specific to the cutaneous tissue being treated. In many cases, complications result from collateral damage created when energy intended for the target tissue is nonselectively diffused to and absorbed by surrounding tissues and structures.
For example, hyperpigmentation and hypopigmentation noted after carbon dioxide laser resurfacing are related to damaged, vaporized melanocytes as well as targeted keratinocytes and fibroblasts in the epidermis and dermis.14 Similarly, unwanted damage to the epidermis may occur during laser-assisted hair removal.
Optometrists who work in conjunction with an aesthetic center should become knowledgeable of the laser procedures offered, the cost of the procedures, and most importantly, the anticipated patient outcomes.
Eyelash Enhancement
Without question, the FDA approval of Latisse in 2008 further solidified our direct involvement in the aesthetic/cosmetic industry. Most of us have had many years of experience with Lumigan (bimatoprost ophthalmic solution 0.03%, Allergan). However, many of us also noticed that this glaucoma medication inadvertently enhanced the size and quality of our patient’s eyelashes. So, it’s no surprise that bimatoprost 0.03% is now FDA approved for treatment of hypotrichosis of the eyelashes.
While the recent splash made by Latisse may not be of the same magnitude as that caused by the debut of Botox, we nonetheless are faced with a sudden demand for this product. Latisse is available by prescription through a pharmacy or can be purchased at a physician’s office, depending on individual state dispensing authority guidelines.
In some states, optometrists have the authority to both prescribe and dispense Latisse. In other states, optometrists can prescribe it, but they cannot dispense/retail it to their patients. In states with a formulary, the state board of optometry may need to approve the addition of Latisse to the formulary before O.D.s can prescribe it. Lastly, optometrists in some states may not be permitted to prescribe it whatsoever.
So, if you do not know if you are permitted to prescribe Latisse, contact your state board of optometry to find out if this indication is within your scope of practice. However, no master list of Latisse-approved states is currently available because state boards are still reviewing their prescribing authority to determine approval for Latisse.
One common question: How extensive of an examination does an optometrist need to perform prior to prescribing Latisse? Considering that the majority of Latisse prescriptions are written by non-eye care providers, it does not appear that an eye exam is absolutely necessary. In my opinion, however, the individual likely should be an active and established patient. As such, you will have a baseline examination and IOP measurement on record. Nonetheless, I do not believe that there is any specific need for a follow-up examination once a patient starts Latisse.
The risks associated with Latisse are the same as those associated with Lumigan—conjunctival hyperemia, skin hyperpigmentation, ocular irritation, dry eye symptoms, change in iris pigmentation and erythema of the eyelid. Generally, these events occur in fewer than 4% of patients.15 Still, remember to use caution when prescribing Latisse for patients with uveitis and patients who are at risk for macular edema, such as aphakes or pseudophakes with a torn posterior capsule.
Today, many optometrists have the option and ability to counsel their patients about a host of cosmetic enhancement procedures. Optometrists who decide to work with cosmetic surgeons should perform appropriate due diligence to ensure the quality of care and customer service is appropriate for their patients. Directly working with a cosmetic surgeon and an aesthetic center can be especially rewarding to your patients and your practice.
Dr. Mann is co-founding partner of SouthEast Eye Specialists, PLLC, an optometric referral center located in Chattanooga, Tenn. Ms. Raymer is director of aesthetics at the Center for Facial Rejuvenation, a medi-spa in Chattanooga opened in 2009 by Dr. Mann and John R. Bierly, M.D. Steven Anderson, M.D., ophthalmic plastic and reconstructive surgeon, serves as the center’s medical director.
1. American Society of Plastic Surgeons. 2009 report of the 2008 statistics, national clearinghouse of plastic surgery statistics. Available at: www.plasticsurgery.org/Media/stats/2008-US-cosmetic-reconstructive-plastic-surgery-minimally-invasive-statistics.pdf (Accessed March 2, 2010).
2. Carruthers J. Botulinum toxin in facial rejuvenation: an update. Dermatol Clin. 2009 Oct;27(4):417-25, v.
3. Shoaib KK, Inam-ul-Haq, Khan MD. Use of botulinum A toxin (BOTOX) in different types of facial dystonia. J Coll Physicians Surg Pak. 2009 Nov;19(11):742-3.
4. Saunders-Pullman R, Soto-Valencia J, Costan-Toth C, et al. A new screening tool for cervical dystonia. Neurology. 2005 Jun 28;64(12):2046-9.
5. Beylot C. Different botulinum toxins and their specifications. Ann Dermatol Venereol. 2009 May;136 Suppl 4:S77-85.
6. Gadhia K, Walmsley AD. Facial aesthetics: is botulinum toxin treatment effective and safe? A systematic review of randomized controlled trials. Br Dent J. 2009 Sep 12;207(5):E9.
7. Baumann L. Collagen-containing fillers: alone and in combination. Clin Plast Surg. 2006 Oct;33(4):587-96.
8. Goldberg DJ. Breakthroughs in US dermal fillers for facial soft-tissue augmentation. J Cosmet Laser Ther. 2009 Dec;11(4):240-7.
9. Fitzgerald R, Vleggaar D. Using poly-L-lactic acid (PLLA) to mimic volume in multiple tissue layers. J Drugs Dermatol. 2009 Oct;8(10 Suppl):s5-14.
10. Ridenour B, Kontis TC. Injectable calcium hydroxylapatite microspheres (Radiesse). Facial Plast Surg. 2009 May;25(2):100-5.
11. Tezel A, Fredrickson GH. The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther. 2008 Mar;10(1):35-42.
12. Weinberg MJ, Solish N. Complications of hyaluronic acid fillers. Facial Plast Surg. 2009 Dec;25(5):324-8.
13. The British Association of Aesthetic Plastic Surgeons (BAAPS). British Association of Aesthetic Plastic Surgeons offers safety guidelines for injectables. Available at: www.baaps.org.uk/about-us/press-releases/491-1-in-4-surgeons-fixing-botched-permanent-filler-ops (Accessed March 2, 2010).
14. Hantash BM, Gladstone HB. Current role of resurfacing lasers. G Ital Dermatol Venereol. 2009 Jun;144(3):229-41.
15. Patil AJ, Vajaranant TS, Edward DP. Bimatoprost - a review. Expert Opin Pharmacother. 2009 Nov;10(16):2759-68.

